1Rutgers New Jersey Medical School, Department of Anesthesia & Perioperative Care, Newark, NJ, USA
ABSTRACT
Pain is the most common reason patients consult primary care providers. According to recent literature, over 40% of the adult Americans, an estimated 100 million people, live with chronic or recurrent pain and pay an estimated $120 billion a year in medical costs in the United States. Continued research is needed to evaluate the impact of pain and the available treatments and determine how to reduce its effect on people’s lives.
A proper treatment strategy should employ therapies providing optimal benefit and minimal harm. Chronic pain, which is categorized as either nociceptive (caused by damage to tissue or inflammatory stimuli) or neuropathic (damage to somatosensory nervous system), is commonly treated by oral opioids. Despite the extensive use of opioids, 40% of pain patients do not achieve adequate pain relief. In addition, the narcotic based, NSAID, and over-the-counter oral medications used for the management of pain is often associated with prolonged healing and undesirable and negative side effects.
There is good evidence that relying on opioids for pain control leads to worse clinical outcomes. Therefore, alternative evidence-based opiate- sparing multimodal approaches of pain management have become increasingly widespread. These therapies include use of combination pharmacotherapy, which target both central and peripheral nociceptive mechanisms, and non-pharmacological interventions. Topical analgesics therapy, including pain relief patches, is nonetheless a potentially valuable strategy in the management of a variety of conditions associated with acute, mild to moderate pain, including acute soft tissue injuries, musculoskeletal pain, and various neuropathic pain disorders.
The purpose of this minimal risk, observational study was to evaluate patients with mild to moderate pain and evaluate perceptions of pain treatment and associated symptoms with the use of a topical painrelieving patch via a subject and clinician reported surveys over the course of two weeks.
Pain is the most common reason patients consult primary care providers. According to recent literature, over 40% of the adult Americans, an estimated 100 million people, live with chronic or recurrent pain and pay an estimated $120 billion a year in medical costs in the United States. Continued research is needed to evaluate the impact of pain and the available treatments and determine how to reduce its effect on people’s lives.
A proper treatment strategy should employ therapies providing optimal benefit and minimal harm. Chronic pain, which is categorized as either nociceptive (caused by damage to tissue or inflammatory stimuli) or neuropathic (damage to somatosensory nervous system), is commonly treated by oral opioids. Despite the extensive use of opioids, 40% of pain patients do not achieve adequate pain relief. In addition, the narcotic based, NSAID, and over-the-counter oral medications used for the management of pain is often associated with prolonged healing and undesirable and negative side effects.
There is good evidence that relying on opioids for pain control leads to worse clinical outcomes. Therefore, alternative evidence-based opiate- sparing multimodal approaches of pain management have become increasingly widespread. These therapies include use of combination pharmacotherapy, which target both central and peripheral nociceptive mechanisms, and non-pharmacological interventions. Topical analgesics therapy, including pain relief patches, is nonetheless a potentially valuable strategy in the management of a variety of conditions associated with acute, mild to moderate pain, including acute soft tissue injuries, musculoskeletal pain, and various neuropathic pain disorders.
The purpose of this minimal risk, observational study was to evaluate patients with mild to moderate pain and evaluate perceptions of pain treatment and associated symptoms with the use of a topical painrelieving patch via a subject and clinician reported surveys over the course of two weeks.
METHODOLOGY
This IRB-approved study evaluated the efficacy of a topical pain-relieving patch containing methyl salicylate 10%, menthol 6% and camphor 3.1% in reducing Brief Pain Inventory (BPI) scores in patients experiencing mild/moderate acute pain.
This IRB-approved study evaluated the efficacy of a topical pain-relieving patch containing methyl salicylate 10%, menthol 6% and camphor 3.1% in reducing Brief Pain Inventory (BPI) scores in patients experiencing mild/moderate acute pain.
- 152 adult patients (100 females, 52 males) with acute arthritic, neurologic or musculoskeletal pain received patches for 14 days.
- A control group of an additional 47 patients (27 females, 20 males) did not receive a patch.
- After 14 days, 34 Patients from the control group crossed over to treatment and were treated with the pain patch.
RESULTS
Paired treatment group data were collected in both active and control groups.
Paired treatment group data were collected in both active and control groups.
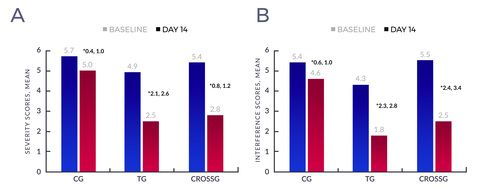
- Over 14 days, treatment group mean BPI Severity score decreased 49% (4.9 to 2.5/10;P<.001, 95% CI [2.09, 2.58] see Figure 1A) and mean BPI Interference score decreased 58% (4.3 to 1.8/10;P<.001, 95% CI [2.33, 2.82] see Figure 1B). Decreases in mean BPI Severity and Interference scores were significantly less in the control group (13%, 15% respectively).
- No side effects of treatment were reported.
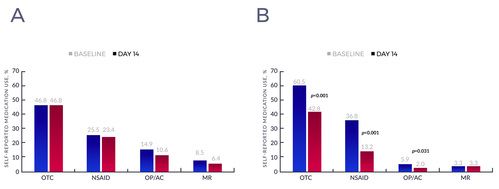
- The total number of patients taking OTC pain medication at baseline in the CG was 22 (46.8%) while in the TG it was 92 (60.5%).
- There was no change in OTC pain medication within the CG; the 22 patients who reported use at baseline also reported use at day 14 (see Figure 2A).
- The number of patients using OTC pain medication decreased by 29.3% in the TG, from 92 patients (60.5%) to 65 patients (42.8%) (see Figure 2B).
- As far as frequency of analgesic use at day 14, 61% of the treatment group were using concomitant oral pain medications "a lot less."
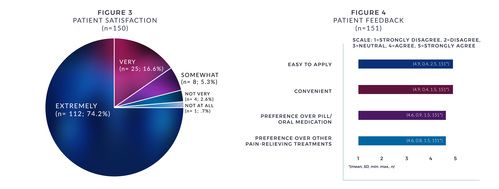
- From a satisfaction standpoint, 90% were "very or extremely" satisfied with the patch (see Figure 3).
- Patients also reported on ease of patch application, convenience, preference over pills/oral medication, and preference over other painrelieving treatments (see Figure 4).
- Importantly, when thirty-four (34) Control Group patients crossed-over to patch treatment, they showed similar reductions in pain severity and interference scores to the Treatment Group.
Figure 1: Baseline and day 14 overall mean (A) severity and (B) interference scores within the control, treatment, and crossover groups. *95% Confidence Interval of the difference, paired t-test. Each difference is statistically significant. Abbreviations: CG, control group; TG, treatment group; CROSSG, crossover group.
Percent of patients using each type of pain medication at baseline and day 14 within the control (A), and treatment (B) groups. Abbreviations: OTC, over-the-counter; NSAID, nonsteroidal anti-inflammatory drug; OP, opioid; AC, anticonvulsant; MR, muscle relaxant.