1Trihealth Cancer Institute, Cincinnati, OH
Long-term PI-based treatment is associated with improved outcomes in MM. Nonetheless, prolonged therapy with parenteral PIs (e.g. bortezomib) can be challenging in the real world, with median duration of therapy (DOT) of 4–7 months. Barriers to this long-term approach may include the burden of repeated intravenous/subcutaneous administration, difficulty travelling to/accessing treatment centers (e.g. due to environmental factors, travel restrictions, social/family situations), patient preference for treatment outside of a hospital or clinic setting, comorbidities, and toxicity. The US MM-6 study (NCT03173092) is investigating in-class transition (iCT) from parenteral bortezomib-based induction to all-oral ixazomib-based therapy (ixazomib-lenalidomide-dexamethasone; IRd) in the diverse US community population with the aim of increasing PI-based treatment duration while maintaining quality of life and improving outcomes. We report updated efficacy and safety for the first 101 patients.
Transplant-ineligible/delayed-transplant (>24 months) NDMM patients with stable disease or better after 3 cycles of bortezomib-based induction are being enrolled at US community sites (including Veterans Affairs hospitals) to receive IRd (ixazomib 4 mg, days 1, 8, 15; lenalidomide 25 mg, days 1–21; dexamethasone 40 mg, days 1, 8, 15, 22) for up to 39 x 28-day cycles or until progression/toxicity. The primary endpoint is progression-free survival (PFS); key secondary endpoints include rates of partial (PR), very good PR (VGPR), and complete response (CR), and DOT.
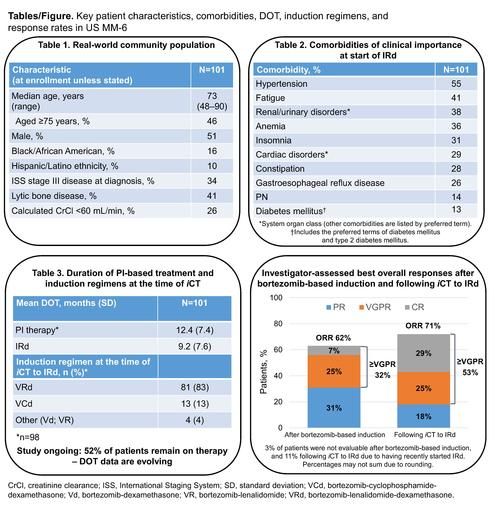
As of June 1 2020, 101 patients had been treated at 21 sites. Median age was 73 years (range 48–90), with 46% aged ≥75 years; 16% and 10% were of African American and Hispanic ethnicity, respectively. Table 1 summarizes the key characteristics of these real-world patients. A total of 95% of patients had ≥1 comorbidity at the start of IRd therapy including renal and urinary disorders (38%), cardiac disorders (29%), peripheral neuropathy (PN; 14%), and diabetes mellitus (13%) (Table 2). With 53 (52%) patients remaining on therapy and enrollment ongoing, mean duration of PI therapy from the start of bortezomib-based induction was 12.4 months, and mean duration of IRd therapy after iCT was 9.2 months (Table 3). Patients have received up to 29.4 months (31 cycles) of IRd to date. The overall response rate (ORR) after bortezomib-based induction was 62% (7% CR, 32% ≥VGPR). After iCT to IRd, the ORR increased to 71%, with the CR and ≥VGPR rates increasing to 29% and 53%, respectively (Figure); of 33 patients with stable disease following bortezomib-based induction, 14 (42%) achieved CR (n=10) or VGPR (n=4) after iCT. With a median follow-up of 12 months and enrollment ongoing, 13 patients had progressed and two had died during PFS analysis. The 12-month PFS rate was 84% (95% CI, 73–91) from the start of bortezomib-based induction and 80% (95% CI, 69–88) from the start of IRd. During IRd treatment to date, 91% of patients have had treatment-emergent adverse events (TEAEs) (54% grade ≥3). Grade 3 TEAEs (≥5% of patients) were diarrhea (8%), pneumonia (7%), and syncope (5%). TEAEs led to study drug modification in 52% of patients and discontinuation in 7% of patients; 37% had serious TEAEs. Diarrhea, nausea, and vomiting occurred in 43%, 23%, and 14% of patients (8%, 2%, 2% grade 3), and led to dose modification in 11%, 5%, and 2%. PN (not elsewhere classified; high-level term) occurred in 32% of patients (2% grade 3) and led to dose modification in 9%. There were three on-study deaths (i.e. occurring <30 days after last dose).
US MM-6 patients reflect the heterogeneous real-world US MM population; the population for this study includes patients from the community who may not be eligible for traditional clinical trials. These updated data in mostly elderly, comorbid, NDMM patients treated in the community setting demonstrate the feasibility and tolerability of iCT to IRd after 3 cycles of bortezomib-based induction; approximately half of patients remain on treatment, and enrollment is ongoing. iCT to IRd resulted in improved responses, with increased rates of ≥VGPR, and prolonged DOT and may thereby improve outcomes for real-world patients. iCT to an all oral regimen could also prevent treatment interruptions for patients who are unable to or prefer not to travel in the context of travel restrictions or other factors.
Disclosures: Girnius: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Yimer: TG Therapeutics: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Sanofi: Speakers Bureau; Texas Oncology: Current Employment; BeiGene: Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Janssen: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Takeda: Speakers Bureau; Celgene, a Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Karyopharm: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; Epizyme: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months. Noga: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Manda: AbbVie: Other: Investigator in AbbVie-sponsored clinical trials. Lyons: Texas Oncology/US Oncology: Current Employment; Novartis: Honoraria. Bogard: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Whidden: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Cherepanov: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Lu: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Aiello: Takeda: Honoraria; Travera: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria. Richter: Celgene: Consultancy, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Speakers Bureau; Janssen: Speakers Bureau; Sanofi: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; AstraZeneca: Consultancy; Secura Bio: Consultancy; Bristol Myers Squibb: Consultancy; X4 Pharmaceuticals: Consultancy; Oncopeptides: Consultancy; Antengene: Consultancy. Rifkin: McKesson: Current equity holder in publicly-traded company, Ended employment in the past 24 months, Other: Stock ownership; Takeda, Amgen, Celgene, BMS, Mylan, Coherus BioSciences, Fresenius: Consultancy; AbbVie: Other: Investigator in AbbVie sponsored clinical trials; Takeda, Amgen, BMS (Celgene): Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure: Real-world evaluation of long-term proteasome inhibition with ixazomib in combination with lenalidomide and dexamethasone for the treatment of newly diagnosed multiple myeloma in non-transplant patients with stable disease after 3 cycles of a bortezomib-based induction.
