1Division of Hematology and Oncology, Weill Cornell Medicine, New York, NY
INTRODUCTION
- Patients with myelodysplastic syndrome (MDS) that is primary refractory to hypomethylating agents (HMAs) have limited treatment options and a median overall survival (OS) of 4-6 months. 1,2
- Inhibition of XPO1 leads to nuclear retention and activation of tumor suppressor proteins (eg, p53, IkB, p21), reduction in oncoprotein mRNAs (c-Myc, Bcl-2, Bcl-6, cyclin D) and selective apoptosis of cancer cells.3
- Recent studies have demonstrated that selinexor, a first generation selective inhibitor of exportin 1 (XPO1, CRM1) mediated nuclear export (SINE), is efficacious in patients with HMA refractory MDS.4
- Eltanexor is an investigational, second-generation oral SINE compound with minimal penetration of the blood brain barrier. The reduced CNS infiltration observed with eltanexor across nonclinical species may confer lower rates of CNS-mediated gastrointestinal toxicity in patients relative to selinexor.
- Following oral administration, animals treated with eltanexor show lower percentage of body weight loss and improved food consumption than animals similarly treated with selinexor. This allows more frequent dosing of eltanexor, enabling a longer period of exposure at higher levels than possible with selinexor.
- In leukemia cells lines, the half-maximal inhibitory value (IC50) is consistently 30% to 50% lower for eltanexor than for selinexor, indicating eltanexor is more active than the first generation XPO1 inhibitor in this setting.
- Eltanexor dosed at 5x/week improved survival relative to selinexor dosed 2x/week in patient-derived xenograft of AML in mouse models (Figure 1).
Figure 1: Non-clinical Model of AML and Eltanexor
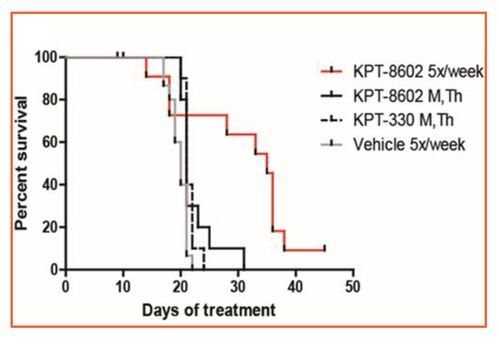
- Eltanexor (KPT-8602) dosed 2x/week showed similar efficacy compared to selinexor (KPT=330).
- Eltanexor dosed 5 days/week showed improved efficacy to selinexor.
STUDY DESIGN AND SCHEMA
- Phase 1/2 open-label study of the safety, tolerability, and efficacy of eltanexor in multiple myeloma, colorectal cancer, metastatic castrate resistant prostate cancer, and higher risk MDS (NCT02649790)
- In the MDS cohort, patients must have documented diagnosis of progressing MDS with 5%-19% myeloblasts
- Patients should have intermediate-2 or high-risk MDS by International Prognostic Scoring System (IPSS)
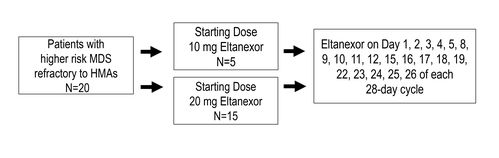
MDS cohort enrolled 20 patients. Demographic characteristics are shown in Table 1.
- 15 patients were administered a starting dose of 20 mg eltanexor
- 5 patients were administered a starting dose of 10 mg eltanexor
- 20 patients were evaluable for safety
- 15 patients were evaluable for efficacy
RESULTS
Table 1: Demographics
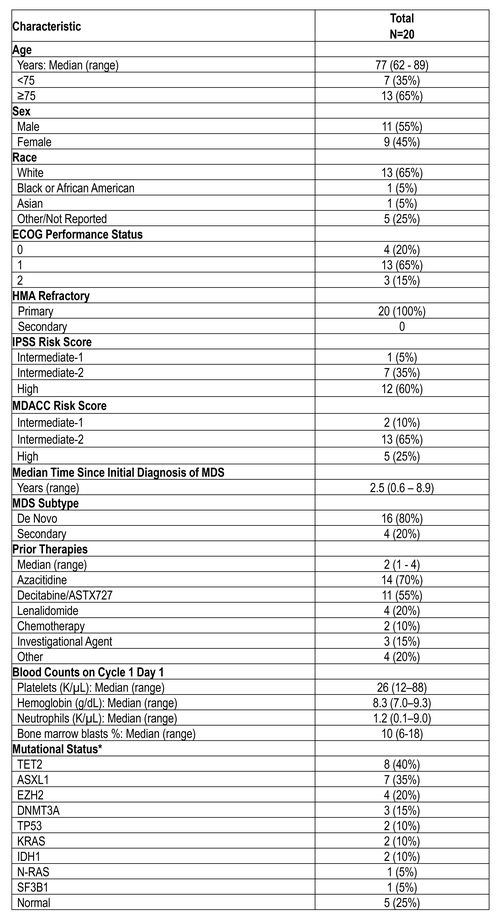
ECOG –Eastern Cooperative Oncology Group, IPSS –International PrognoticScoring System, MDACC –MD Anderson Cancer Center
*Patients may have more than one mutation or cytogenetic abnormality
*Patients may have more than one mutation or cytogenetic abnormality
Table 2: Treatment-Related Adverse Events (≥10%)
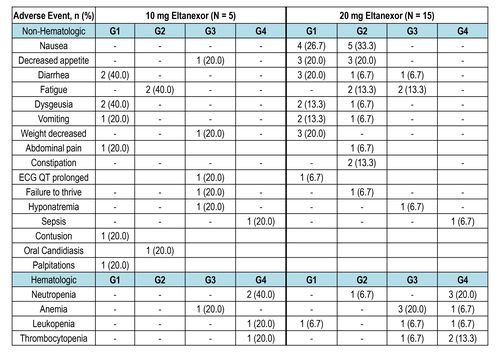
Table 3: Dose Reduction
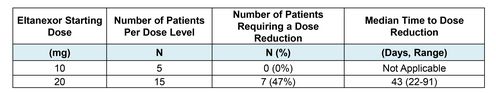
- Starting dose of 20 mg in patients with MDS was based on recommend Phase 2 dose determined in a dose escalation phase of study in patients with relapsed/refractory multiple myeloma.
- Because dose was reduced to 10 mg for nearly half of patients initially treated with 20 mg, a second cohort of patients (n=5) with MDS was enrolled at a starting dose of 10 mg to reduce AEs and improve tolerability.
Table 4: Efficacy
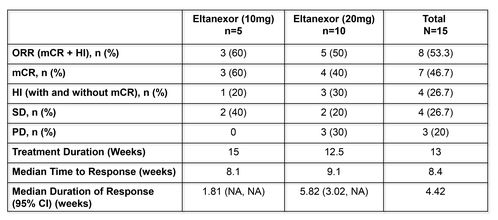
HI = hematologic improvement (minimum of 8 weeks); mCR = marrow complete remission; NA = notavailable; ORR= overall response rate including CR, PR, mCR and HI; PD = progressive disease; PR =
partial response; SD = stable disease
partial response; SD = stable disease
- In the 10-mg cohort (n=5), all patients derived clinical benefit: 3 patients (60%) reached mCR and 2 patients (40%) SD.
- In the 20-mg cohort (n=10), 4 patients (40%) reached mCR and 2 (20%) SD.
- Four patients had HI and became transfusion independent for at least 8 weeks, including 2 patients with tri-lineage HI.
Figure 2: Time on Treatment
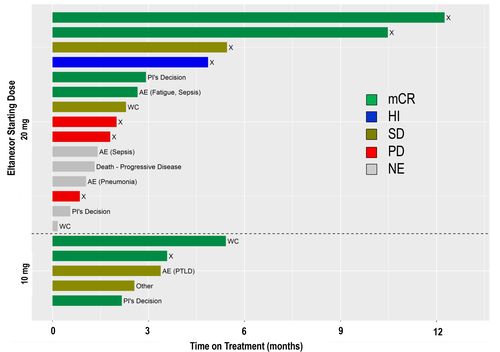
AE=adverse event, HI=hematologic improvement (minimum of 8 weeks), mCR=marrow complete response, NE=non-evaluable, PD=progressive disease, PI=Principal Investigator, PTLD=post transplant lymphoproliferative
disorder, SD=stable disease, WC=withdrawal of consent, X=off treatment
disorder, SD=stable disease, WC=withdrawal of consent, X=off treatment
Figure 3: Overall Survival
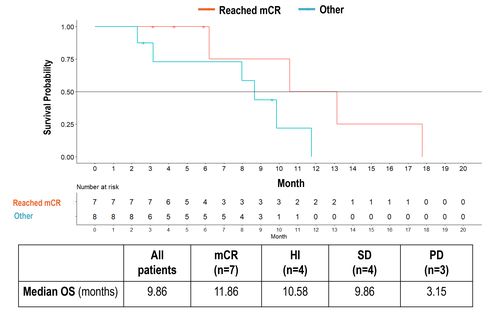
- Median OS mCR vs Other: 11.86 vs 8.67 months (HR=0.27, p=0.05)
- Median OS mCR vs PD: 11.86 vs 3.15 months (HR=0.23, p=0.04)
CONCLUSIONS
• Single-agent oral eltanexor was active in patients with high-risk MDS that is
primary refractory to HMAs.
• Patients with mCR had significantly longer mOS than patients without mCR or
with PD.
• Further evaluation of eltanexor in MDS as a single agent and in combination with
other agents is ongoing.
• Single-agent oral eltanexor was active in patients with high-risk MDS that is
primary refractory to HMAs.
• Patients with mCR had significantly longer mOS than patients without mCR or
with PD.
• Further evaluation of eltanexor in MDS as a single agent and in combination with
other agents is ongoing.
