TPS171: 18F-Fluciclovine positron emission tomography (PET) in metastatic castration-resistant prostate cancer (mCRPC)
1Tulane University School of Medicine, New Orleans, LA
BACKGROUND
Conventional imaging of prostate cancer has limitations in staging, restaging after biochemical relapse, and response assessment. Functional imaging with positron emission tomography (PET) can target various aspects of tumor biology and has shown to be superior in the detection of prostate cancer compared with conventional computed tomography (CT) and bone scans.
18F-Fluciclovine, a synthetic amino acid transported across mammalian cell membranes by amino acid transporters that is upregulated to a greater extent in prostate cancer cells than in surrounding tissue, is currently approved for PET imaging for patients with biochemical recurrence.
The role of 18F-fluciclovine PET scans in monitoring response to novel hormonal therapies such as abiraterone acetate is unclear. We hypothesize that 1) using 18F-fluciclovine PET scanning will allow a more sensitive assessment of mCRPC patients at the initiation of systemic therapy with abiraterone acetate and 2) the changes observed in 18F-fluciclovine PET will correlate better with the serologic changes in PSA, allowing superior disease monitoring, than conventional imaging modalities.
Conventional imaging of prostate cancer has limitations in staging, restaging after biochemical relapse, and response assessment. Functional imaging with positron emission tomography (PET) can target various aspects of tumor biology and has shown to be superior in the detection of prostate cancer compared with conventional computed tomography (CT) and bone scans.
18F-Fluciclovine, a synthetic amino acid transported across mammalian cell membranes by amino acid transporters that is upregulated to a greater extent in prostate cancer cells than in surrounding tissue, is currently approved for PET imaging for patients with biochemical recurrence.
The role of 18F-fluciclovine PET scans in monitoring response to novel hormonal therapies such as abiraterone acetate is unclear. We hypothesize that 1) using 18F-fluciclovine PET scanning will allow a more sensitive assessment of mCRPC patients at the initiation of systemic therapy with abiraterone acetate and 2) the changes observed in 18F-fluciclovine PET will correlate better with the serologic changes in PSA, allowing superior disease monitoring, than conventional imaging modalities.
METHOD
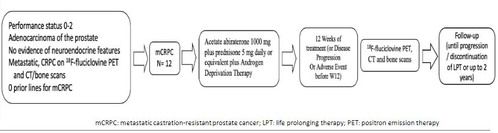
This single-arm, pilot study (NCT04158245) will describe the changes in 18F-fluciclovine PET scans and compare these results with PSA and conventional computerized tomography (CT) and bone scans, in mCRPC patients treated with abiraterone acetate plus prednisone.
The co-primary objectives of the study include the 18F-fluciclovine PET changes at baseline and 12 weeks after abiraterone acetate for mCRPC and the comparison between 18F-fluciclovine PET and conventional scans.
Secondary and exploratory endpoints include PSA response, PSA progression and genomic alterations by next-generation sequencing. As of 12 September 2020, this trial is actively enrolling.
Study Population:
Patients must have a detectable baseline PSA of ≥ 2 ng/mL and metastatic, castration-resistant prostate cancer with detected metastases on conventional CT and bone scans.
Treatment Plan:
Twelve patients will be treated with abiraterone 1000 mg daily plus prednisone 5 mg (or dexamethasone 0.5 mg) daily for mCRPC and get 18F-fluciclovine PET and conventional CT and bone scans at baseline and 12 weeks after starting abiraterone therapy or at disease progression.
PSA progression will be defined as a repeated increase in PSA of at least 2 ng/dL and 25% from nadir values, at least 1 week apart, according to PCWG3 criteria and clinical or radiographic progression by RECIST version 1.1.
This single-arm, pilot study (NCT04158245) will describe the changes in 18F-fluciclovine PET scans and compare these results with PSA and conventional computerized tomography (CT) and bone scans, in mCRPC patients treated with abiraterone acetate plus prednisone.
The co-primary objectives of the study include the 18F-fluciclovine PET changes at baseline and 12 weeks after abiraterone acetate for mCRPC and the comparison between 18F-fluciclovine PET and conventional scans.
Secondary and exploratory endpoints include PSA response, PSA progression and genomic alterations by next-generation sequencing. As of 12 September 2020, this trial is actively enrolling.
Study Population:
Patients must have a detectable baseline PSA of ≥ 2 ng/mL and metastatic, castration-resistant prostate cancer with detected metastases on conventional CT and bone scans.
Treatment Plan:
Twelve patients will be treated with abiraterone 1000 mg daily plus prednisone 5 mg (or dexamethasone 0.5 mg) daily for mCRPC and get 18F-fluciclovine PET and conventional CT and bone scans at baseline and 12 weeks after starting abiraterone therapy or at disease progression.
PSA progression will be defined as a repeated increase in PSA of at least 2 ng/dL and 25% from nadir values, at least 1 week apart, according to PCWG3 criteria and clinical or radiographic progression by RECIST version 1.1.
RESULT
A total of 316 patients with ccRCC, median age 62 (range 32-90), 71.8% men, were included. Tissue samples were obtained from primary tumor (46.5%), lung (12.3%), bone (9.5%), liver (4.7%) and other metastatic sites (27%). Gene expression analysis identified angiogenic, mixed and T-effector subgroups in 24.1%, 51.3% and 24.7%, respectively. Patients with angiogenic subgroup tumors compared to those with T-effector subgroup tumors were more likely to be older (63 versus 60 years, p=0.035), female (40.8% versus 16.7%, p=0.0009) and more frequently found in pancreatic/small bowel metastases (75% versus 12.5%, p=0.0103).
Biomarkers of potential response to immunotherapy such as PD-L1 (p=0.0021), TMB (not significant), and dMMR/MSI-H status (not significant) were more frequent in the T-effector subgroup. PBRM1 mutations were more common in the angiogenic subgroup (62.0% vs 37.5%, p=0.0034) while BAP1 mutations were more common in the T-effector subgroup (18.6% versus 3.0%, p= 0.0035). Immune cell population abundance (e.g. NK cells, monocytes) and immune checkpoint gene expression (TIM-3, PD-L1, PD-L2, CTLA4) were also increased in the T-effector subgroup.
A total of 316 patients with ccRCC, median age 62 (range 32-90), 71.8% men, were included. Tissue samples were obtained from primary tumor (46.5%), lung (12.3%), bone (9.5%), liver (4.7%) and other metastatic sites (27%). Gene expression analysis identified angiogenic, mixed and T-effector subgroups in 24.1%, 51.3% and 24.7%, respectively. Patients with angiogenic subgroup tumors compared to those with T-effector subgroup tumors were more likely to be older (63 versus 60 years, p=0.035), female (40.8% versus 16.7%, p=0.0009) and more frequently found in pancreatic/small bowel metastases (75% versus 12.5%, p=0.0103).
Biomarkers of potential response to immunotherapy such as PD-L1 (p=0.0021), TMB (not significant), and dMMR/MSI-H status (not significant) were more frequent in the T-effector subgroup. PBRM1 mutations were more common in the angiogenic subgroup (62.0% vs 37.5%, p=0.0034) while BAP1 mutations were more common in the T-effector subgroup (18.6% versus 3.0%, p= 0.0035). Immune cell population abundance (e.g. NK cells, monocytes) and immune checkpoint gene expression (TIM-3, PD-L1, PD-L2, CTLA4) were also increased in the T-effector subgroup.
CONCLUSION
Our hierarchical clustering results based on the 66-gene expression signature were concordant with results from prior studies. Patient subgroups identified by evaluation of angiogenic and T-effector signature scores exhibit significantly different mutations and immune profiles. These findings require prospective validation in future biomarker-selected clinical trials.
Our hierarchical clustering results based on the 66-gene expression signature were concordant with results from prior studies. Patient subgroups identified by evaluation of angiogenic and T-effector signature scores exhibit significantly different mutations and immune profiles. These findings require prospective validation in future biomarker-selected clinical trials.
DISCLOSURES
-
-
The data in this poster was presented atASCO GU 2021. Published with permission from the Copyright owner.
