ABSTRACT
Thrombocytopenia, a reduction of circulating platelets, can be caused by decreased platelet production or increased platelet destruction. A biomarker which can rapidly assess the aetiology of thrombocytopenia would be beneficial to clinicians. Sysmex XN analysers can measure reticulated platelets, expressed as immature platelet fraction (IPF%). This parameter may also predict platelet recovery allowing for justification of platelet transfusions prophylactically. A normal reference range for IPF% of 1.1-8.5%was established using healthy controls (n=100). 87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). IPF% was significantly higher in those with increased platelet destruction (p<0.05). Patients with immune thrombocytopenia purpura (ITP) had a significantly higher IPF% (13.7%) than aplastic anaemia (1.9%) and control patients (3.8%) (p<0.05). ROC curve analysis determined a diagnostic cut off point of 6.8% with a sensitivity of 87% and specificity of 92.9%. 14 patients were followed daily for platelet recovery through platelet count and IPF%. Platelet recovery occurred within two days of an increase in IPF% of 2.1% or greater from platelet nadir (sensitivity 92%, specificity 96%, p<0.05). IPF% measured on the Sysmex XN analysers could be a useful clinical parameter in the evaluation of patients presenting with thrombocytopenia potentially avoiding invasive procedures such as bone marrow examination. IPF% can also predict platelet recovery leading to rationalisation of prophylactic platelet transfusions. This rapid, inexpensive parameter should be implemented into routine full blood count analysis allowing for thorough evaluationof thrombocytopenic patients.
Thrombocytopenia, a reduction of circulating platelets, can be caused by decreased platelet production or increased platelet destruction. A biomarker which can rapidly assess the aetiology of thrombocytopenia would be beneficial to clinicians. Sysmex XN analysers can measure reticulated platelets, expressed as immature platelet fraction (IPF%). This parameter may also predict platelet recovery allowing for justification of platelet transfusions prophylactically. A normal reference range for IPF% of 1.1-8.5%was established using healthy controls (n=100). 87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). IPF% was significantly higher in those with increased platelet destruction (p<0.05). Patients with immune thrombocytopenia purpura (ITP) had a significantly higher IPF% (13.7%) than aplastic anaemia (1.9%) and control patients (3.8%) (p<0.05). ROC curve analysis determined a diagnostic cut off point of 6.8% with a sensitivity of 87% and specificity of 92.9%. 14 patients were followed daily for platelet recovery through platelet count and IPF%. Platelet recovery occurred within two days of an increase in IPF% of 2.1% or greater from platelet nadir (sensitivity 92%, specificity 96%, p<0.05). IPF% measured on the Sysmex XN analysers could be a useful clinical parameter in the evaluation of patients presenting with thrombocytopenia potentially avoiding invasive procedures such as bone marrow examination. IPF% can also predict platelet recovery leading to rationalisation of prophylactic platelet transfusions. This rapid, inexpensive parameter should be implemented into routine full blood count analysis allowing for thorough evaluationof thrombocytopenic patients.
INTRODUCTION
The main causes of thrombocytopenia can be divided into two groups; a failure in the productions of platelets (e.g. aplastic anaemia) and increased peripheral platelet destruction (e.g. ITP). Patients with haematological malignancies more commonly present with thrombocytopenia than any other cohort. Early identification of the cause of thrombocytopenia is important to provide timely intervention to decrease the risk of life-threatening bleeding. Such intervention includes platelet transfusion support. The main reason for justification of platelet transfusion to thrombocytopenic patients is platelet count in correlation with clinical evaluation of the patient. Sysmex XN analysers allow for the measurement of immature platelet fraction (IPF%), differentiating mature from immature platelets. IPF% has the ability to distinguish between the causes of thrombocytopenia, differentiating decreased platelet production from increased platelet destruction. IPF% also has the ability to predict platelet recovery, reducing unnecessary platelet transfusions in patients where the platelet count would recover without intervention.
The main causes of thrombocytopenia can be divided into two groups; a failure in the productions of platelets (e.g. aplastic anaemia) and increased peripheral platelet destruction (e.g. ITP). Patients with haematological malignancies more commonly present with thrombocytopenia than any other cohort. Early identification of the cause of thrombocytopenia is important to provide timely intervention to decrease the risk of life-threatening bleeding. Such intervention includes platelet transfusion support. The main reason for justification of platelet transfusion to thrombocytopenic patients is platelet count in correlation with clinical evaluation of the patient. Sysmex XN analysers allow for the measurement of immature platelet fraction (IPF%), differentiating mature from immature platelets. IPF% has the ability to distinguish between the causes of thrombocytopenia, differentiating decreased platelet production from increased platelet destruction. IPF% also has the ability to predict platelet recovery, reducing unnecessary platelet transfusions in patients where the platelet count would recover without intervention.
METHOD
IPF% is available as a parameter on the Sysmex XN haematology analysers and measure on K2EDTA samples. 87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). Patients with normal FBC parameters (n=100) were included to establish a reference range for IPF%, represented using the 2.5thand 97.5thpercentile. Comparison of IPF% amongst each group was carried out using Mann-Whitney U test. Reproducibility of IPF% was assessed by coefficient of variation (CV%) analysis. 14 patients were followed daily through platelet count and IPF% to determine the ability of IPF% to predict platelet recovery. ROC curve analysis was performed to determine the diagnostic cut off point of IPF% in differentiating between ITP and aplastic anaemia. ROC curve analysis was also used to determine the optimal value of IPF% for the prediction of platelet recovery.
IPF% is available as a parameter on the Sysmex XN haematology analysers and measure on K2EDTA samples. 87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). Patients with normal FBC parameters (n=100) were included to establish a reference range for IPF%, represented using the 2.5thand 97.5thpercentile. Comparison of IPF% amongst each group was carried out using Mann-Whitney U test. Reproducibility of IPF% was assessed by coefficient of variation (CV%) analysis. 14 patients were followed daily through platelet count and IPF% to determine the ability of IPF% to predict platelet recovery. ROC curve analysis was performed to determine the diagnostic cut off point of IPF% in differentiating between ITP and aplastic anaemia. ROC curve analysis was also used to determine the optimal value of IPF% for the prediction of platelet recovery.
RESULT
IPF% as a Diagnostic Marker
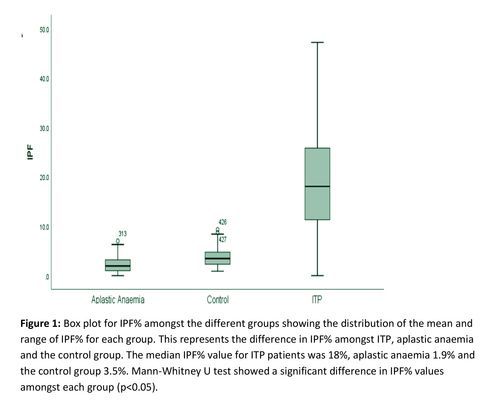
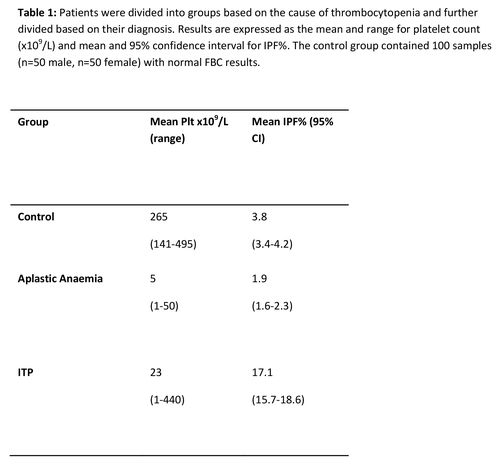
IPF% was assessed in patients with ITP, aplastic anaemia and a control group. A statistically significant different in IPF% between ITP and AA patients (p<0.05) and the control group (p<0.05) was found. ROC curve analysis determined a cut of point of 6.8% for differentiating ITP from AA with a sensitivity of 87% and specificity of 92.9%. (Figure 1)(Table 1)
IPF% as a Diagnostic Marker
IPF% was assessed in patients with ITP, aplastic anaemia and a control group. A statistically significant different in IPF% between ITP and AA patients (p<0.05) and the control group (p<0.05) was found. ROC curve analysis determined a cut of point of 6.8% for differentiating ITP from AA with a sensitivity of 87% and specificity of 92.9%. (Figure 1)(Table 1)
IPF% in Predicting Platelet Recovery
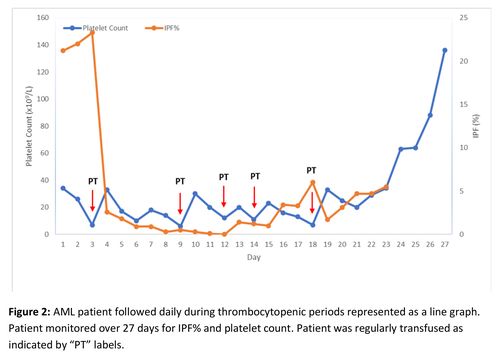
To determine the sensitivity and specificity of IPF% in predicting imminent platelet recovery, ROC curve analysis was used. The increase in IPF% for ROC curve analysis was defined as the increase in IPF% from platelet nadir to the peak IPF% prior to platelet recovery. An increase of 2.1% was found to have a sensitivity and specificity of 92% and 96% respectively in predicting platelet recovery within 2 days of increase in IPF%. (Figure 2)
To determine the sensitivity and specificity of IPF% in predicting imminent platelet recovery, ROC curve analysis was used. The increase in IPF% for ROC curve analysis was defined as the increase in IPF% from platelet nadir to the peak IPF% prior to platelet recovery. An increase of 2.1% was found to have a sensitivity and specificity of 92% and 96% respectively in predicting platelet recovery within 2 days of increase in IPF%. (Figure 2)
CONCLUSION
IPF% results, easily available on the Sysmex XN analysers, could be a useful clinical parameter in the evaluation of patients presenting with thrombocytopenia potentially avoiding invasive procedures such as bone marrow examination. IPF% can also predict platelet recovery leading to rationalisation of prophylactic platelet transfusions. This rapid, inexpensive parameter should be implemented into routine full blood count analysis allowing for thorough evaluation of thrombocytopenic patients.
IPF% results, easily available on the Sysmex XN analysers, could be a useful clinical parameter in the evaluation of patients presenting with thrombocytopenia potentially avoiding invasive procedures such as bone marrow examination. IPF% can also predict platelet recovery leading to rationalisation of prophylactic platelet transfusions. This rapid, inexpensive parameter should be implemented into routine full blood count analysis allowing for thorough evaluation of thrombocytopenic patients.
The data in this poster was presented at EHA 2021. Published with permission from the Copyright owner.
REFERENCES
1. Briggs. C, Kunka. S, Hart. D, Oguni. S, Machi. S. (2006) Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoetic stem cell transplantation. Transfusion Medicine
2. Pons. I, Monteagudo. M, Lucchetti. G, Munoz. L, Perea. G, Colomina. I, Obiols. J. (2010). Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. European Journal of Haematology.
1. Briggs. C, Kunka. S, Hart. D, Oguni. S, Machi. S. (2006) Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoetic stem cell transplantation. Transfusion Medicine
2. Pons. I, Monteagudo. M, Lucchetti. G, Munoz. L, Perea. G, Colomina. I, Obiols. J. (2010). Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. European Journal of Haematology.
