ABSTRACT
Background: Multi-tyrosine kinase inhibitors(TKIs)were until recently, the only available drugs to treat HCC. However, their use was often limited due to the adverse effects profile. For this unmet need, nivolumaburged asa second-line treatment for patients with advanced HCC and functional hepatic reserve, who progressed to or did not tolerate TKIs. Although the phase III clinical trial did not achieve its primary endpoint, durable responses were seen. Thus, this study aims to evaluate the safety profile of nivolumab in HCC in a real-world setting. Method: Retrospective and multicenter study of advanced HCC treated with nivolumab between January 2017 and December 2020in 5 tertiary hospitals. SPSS was used for data analysis. Results: Twenty-sixpatients were included, with a median age of 60 years (61-24 years). Cirrhosis was present in 17 patients (65%) and extra-hepatic involvement in 10 (37%). According to C-P score, at the start of nivolumab, 19 (73%) and 7 (27%) patients were A and B score, respectively. Nivolumab was 1st, 2ndand 3rdline in 3 patients, 17 and in 6 patients, respectively. Treatment related adverse eventswere seen in 16 patients (59%), 3 grade 3 (14%) and 1 grade 4 (bleeding) event. The most common adverse events were asthenia, hepatic and gastrointestinal toxicity. Fifteen deaths (58%) were observed. The median overall survival(OS)was 101 [36-178], 182[121–188] and 195weeks [87-209], forthe1st, 2ndand 3rdline treatment.A C-P score of A at the start of nivolumab was related with superior OS (P=0.014).The medianfollow-up time was 159 weeks. Conclusion: The survival of patients with advanced HCC treated with nivolumab was related to C-Pscore at the start of treatment. Nivolumab is an option to consider with a favorable safety profile. Prospective studies are warranted.
Background: Multi-tyrosine kinase inhibitors(TKIs)were until recently, the only available drugs to treat HCC. However, their use was often limited due to the adverse effects profile. For this unmet need, nivolumaburged asa second-line treatment for patients with advanced HCC and functional hepatic reserve, who progressed to or did not tolerate TKIs. Although the phase III clinical trial did not achieve its primary endpoint, durable responses were seen. Thus, this study aims to evaluate the safety profile of nivolumab in HCC in a real-world setting. Method: Retrospective and multicenter study of advanced HCC treated with nivolumab between January 2017 and December 2020in 5 tertiary hospitals. SPSS was used for data analysis. Results: Twenty-sixpatients were included, with a median age of 60 years (61-24 years). Cirrhosis was present in 17 patients (65%) and extra-hepatic involvement in 10 (37%). According to C-P score, at the start of nivolumab, 19 (73%) and 7 (27%) patients were A and B score, respectively. Nivolumab was 1st, 2ndand 3rdline in 3 patients, 17 and in 6 patients, respectively. Treatment related adverse eventswere seen in 16 patients (59%), 3 grade 3 (14%) and 1 grade 4 (bleeding) event. The most common adverse events were asthenia, hepatic and gastrointestinal toxicity. Fifteen deaths (58%) were observed. The median overall survival(OS)was 101 [36-178], 182[121–188] and 195weeks [87-209], forthe1st, 2ndand 3rdline treatment.A C-P score of A at the start of nivolumab was related with superior OS (P=0.014).The medianfollow-up time was 159 weeks. Conclusion: The survival of patients with advanced HCC treated with nivolumab was related to C-Pscore at the start of treatment. Nivolumab is an option to consider with a favorable safety profile. Prospective studies are warranted.
INTRODUCTION
The management of hepatocellular carcinoma (HCC) is hampered by the presence of underlyingliver diseaseand until recently,multi-tyrosine kinase inhibitors(TKIs)were the only available drugs totreat HCC. However, their usewas often limited in clinical practice due to the adverse effects profile and the potential to deteriorate baselineliver function. For this unmet need, FDA approved nivolumabas a second-line treatment for patients with advanced HCC and Child-Pugh (C-P)liver function score ≤ B, who progressed to or did not tolerate TKIs. Although the phase III clinical trial did not achieve its primary endpoint, partial and durable responses were seen. Thus, this study aims to evaluate the safety profile of nivolumab in HCC in a real-world setting.
The management of hepatocellular carcinoma (HCC) is hampered by the presence of underlyingliver diseaseand until recently,multi-tyrosine kinase inhibitors(TKIs)were the only available drugs totreat HCC. However, their usewas often limited in clinical practice due to the adverse effects profile and the potential to deteriorate baselineliver function. For this unmet need, FDA approved nivolumabas a second-line treatment for patients with advanced HCC and Child-Pugh (C-P)liver function score ≤ B, who progressed to or did not tolerate TKIs. Although the phase III clinical trial did not achieve its primary endpoint, partial and durable responses were seen. Thus, this study aims to evaluate the safety profile of nivolumab in HCC in a real-world setting.
MATERIALS AND METHODS
Retrospective and multicenter study of patients with advanced HCC treated with nivolumab between January 2017 and December 2020in 5 tertiary hospitals. SPSS was used for data analysis.Atotal of 26 pts with advanced HCC submitted to at least 1 cycle of nivolumabwere included.
Retrospective and multicenter study of patients with advanced HCC treated with nivolumab between January 2017 and December 2020in 5 tertiary hospitals. SPSS was used for data analysis.Atotal of 26 pts with advanced HCC submitted to at least 1 cycle of nivolumabwere included.
RESULT
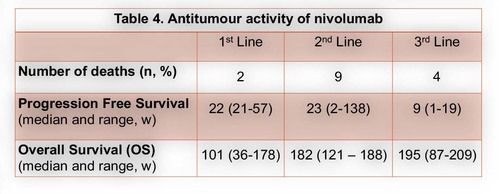
From January 2017 to December 2020, a total of 26 pts with advanced HCC were submitted to at least 1 cycle of nivolumab.Themedian age was60 years (61-24 years)and 25 patients (96%) male gender. Chronic liver disease was due to alcohol in 12 patients (44%), HCV infection in 6 (22%) and HCV and HBV co-infection in 3 (11%). Cirrhosis was present in 17 patients (65%), portal hypertension stigma in 13 (48%)and extra-hepatic involvement in 10 (37%). According to C-P score, at the start of nivolumab, 19 (73%) and 7 (27%) patients were A and B score, respectively. Sorafenib was first line in 23 patients (88%) and nivolumab in 3 (12%). Regarding to second-line treatment, nivolumab was an option in 17 patients (74%) and regorafenib in 6 (26%). Six patients started a third-line with nivolumab, 5 with an A C-P score. Under CTCAE, 22 treatment related adverse eventswere seen in 16 patients (59%), 3 grade 3 (14%) and 1 grade 4 (bleeding) event. The most common adverse events were asthenia, hepatic and gastrointestinal toxicity.More detailed information in table 3.Toxicity leading to definitive suspension was seen in 6 patients (1 bleeding event, 1 hepatic toxicity,1 heart failure and 2 with grade 2 persistent asthenia). Disease controlwas achieved in 9 patients (37%), with 1 partial response.In the first-,second-and third-linesetting the median time to progressionfrom the start of immunotherapywas 22[21-67], 23 [2–138] and 9weeks [1-19], respectively. Fifteen deaths (58%) were observed, 9 in second-and 4 inthethird-line. The median overall survival (OS) was 101 [36-178], 182[121–188] and 195weeks [87-209], forthefirst, second-and third-line treatment.A C-P score of A at the start of nivolumab was related with superior OS (P=0.014).The medianfollow-up time was 159 weeks, with 6 patients under nivolumab at the end of the study, 2 in the third line setting. One patient with no disease progression, one year after suspension of treatment.
From January 2017 to December 2020, a total of 26 pts with advanced HCC were submitted to at least 1 cycle of nivolumab.Themedian age was60 years (61-24 years)and 25 patients (96%) male gender. Chronic liver disease was due to alcohol in 12 patients (44%), HCV infection in 6 (22%) and HCV and HBV co-infection in 3 (11%). Cirrhosis was present in 17 patients (65%), portal hypertension stigma in 13 (48%)and extra-hepatic involvement in 10 (37%). According to C-P score, at the start of nivolumab, 19 (73%) and 7 (27%) patients were A and B score, respectively. Sorafenib was first line in 23 patients (88%) and nivolumab in 3 (12%). Regarding to second-line treatment, nivolumab was an option in 17 patients (74%) and regorafenib in 6 (26%). Six patients started a third-line with nivolumab, 5 with an A C-P score. Under CTCAE, 22 treatment related adverse eventswere seen in 16 patients (59%), 3 grade 3 (14%) and 1 grade 4 (bleeding) event. The most common adverse events were asthenia, hepatic and gastrointestinal toxicity.More detailed information in table 3.Toxicity leading to definitive suspension was seen in 6 patients (1 bleeding event, 1 hepatic toxicity,1 heart failure and 2 with grade 2 persistent asthenia). Disease controlwas achieved in 9 patients (37%), with 1 partial response.In the first-,second-and third-linesetting the median time to progressionfrom the start of immunotherapywas 22[21-67], 23 [2–138] and 9weeks [1-19], respectively. Fifteen deaths (58%) were observed, 9 in second-and 4 inthethird-line. The median overall survival (OS) was 101 [36-178], 182[121–188] and 195weeks [87-209], forthefirst, second-and third-line treatment.A C-P score of A at the start of nivolumab was related with superior OS (P=0.014).The medianfollow-up time was 159 weeks, with 6 patients under nivolumab at the end of the study, 2 in the third line setting. One patient with no disease progression, one year after suspension of treatment.
Figure 1
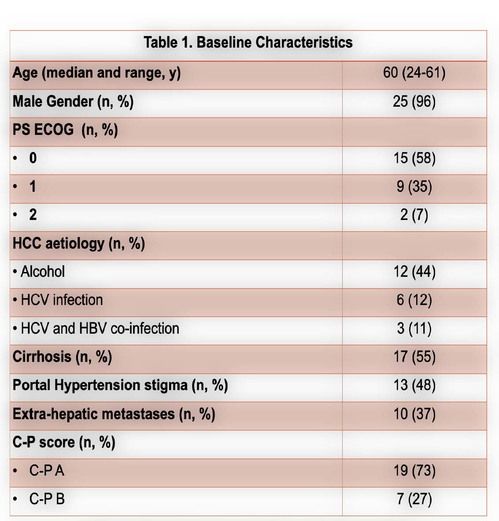
Figure 2
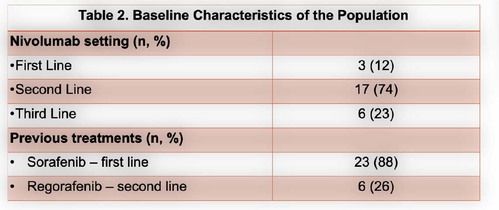
Figure 3
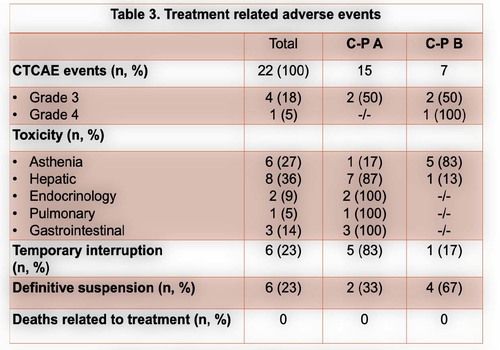
Figure 4
CONCLUSION
The survival of patients with advanced HCC treated with nivolumab was related to C-P score at the start of treatment. Nivolumab is an option to consider with a favorable safety profile consistent with previous reports. Prospective studies are warranted.
The survival of patients with advanced HCC treated with nivolumab was related to C-P score at the start of treatment. Nivolumab is an option to consider with a favorable safety profile consistent with previous reports. Prospective studies are warranted.
REFERENCES
1- B El-Khoueiry A. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 2492-2502.
2- 2-Finn R., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New England Journal of Medicine 2020; 382:1894-190.
1- B El-Khoueiry A. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 2492-2502.
2- 2-Finn R., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New England Journal of Medicine 2020; 382:1894-190.
The data in this poster was presented at World Congress on Gastrointestinal Cancer 2021. Published with permission from the Copyright owner.
