1Haemopoiesis Research Laboratory, University of Crete School of Medicine, Heraklion, Greece;
INTRODUCTION
Patients with persistent, unexplained neutropenia who do not fulfill the diagnostic criteria of any underlying disease are characterized as chronic idiopathic neutropenia (CIN) cases [1]. Real-world data based on longitudinal studies of large number of CIN patients are rare. A cooperation in science and technology (COST) Action, the EuNet-INNOCHRON, has been recently launched, aiming to promote the research on chronic neutropenias, including CIN, across and beyond Europe. We present longitudinal laboratory and clinical data from adult patients with CIN registered in a database within EuNet-INNOCHRON aiming to provide insights in the natural history and outcome of patients with this rare disease.
Patients with persistent, unexplained neutropenia who do not fulfill the diagnostic criteria of any underlying disease are characterized as chronic idiopathic neutropenia (CIN) cases [1]. Real-world data based on longitudinal studies of large number of CIN patients are rare. A cooperation in science and technology (COST) Action, the EuNet-INNOCHRON, has been recently launched, aiming to promote the research on chronic neutropenias, including CIN, across and beyond Europe. We present longitudinal laboratory and clinical data from adult patients with CIN registered in a database within EuNet-INNOCHRON aiming to provide insights in the natural history and outcome of patients with this rare disease.
MATERIALS AND METHODS
Patient data are registered in the “NeutroReg” electronic registry hosted in the University of Crete Data Center ιn compliance with the European General Data Protection Regulation. The enrollment is based on previously described diagnostic criteria for CIN, i.e. absolute neutrophil counts (ANC) below 1800/μl of blood for more than 6 months and no evidence of any underlying disease/condition associated with neutropenia following a defined clinical/laboratory investigation including bone marrow biopsy and karyotype. In all registered patients we performed targeted next generation sequencing (NGS) of myeloid genes for identification of clonal hemopoiesis [2] [Figure 1] (MiSeq, Illumina) and genotyping of the DARC/ACKR1 gene for identification of the single nucleotide polymorphism (SNP) rs2814778 (-46T>C) associated with benign ethnic neutropenia [3].
Patient data are registered in the “NeutroReg” electronic registry hosted in the University of Crete Data Center ιn compliance with the European General Data Protection Regulation. The enrollment is based on previously described diagnostic criteria for CIN, i.e. absolute neutrophil counts (ANC) below 1800/μl of blood for more than 6 months and no evidence of any underlying disease/condition associated with neutropenia following a defined clinical/laboratory investigation including bone marrow biopsy and karyotype. In all registered patients we performed targeted next generation sequencing (NGS) of myeloid genes for identification of clonal hemopoiesis [2] [Figure 1] (MiSeq, Illumina) and genotyping of the DARC/ACKR1 gene for identification of the single nucleotide polymorphism (SNP) rs2814778 (-46T>C) associated with benign ethnic neutropenia [3].
Figure 1
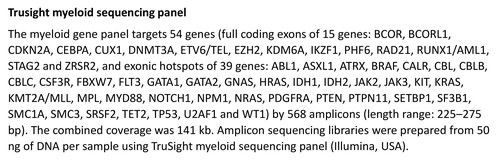
Figure 2
RESULTS AND DISCUSSION
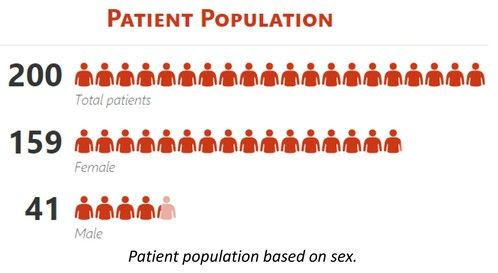
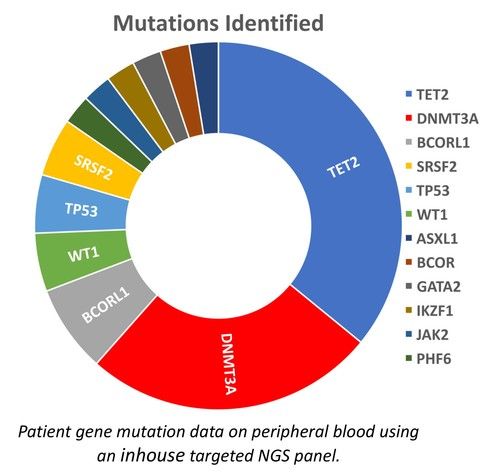
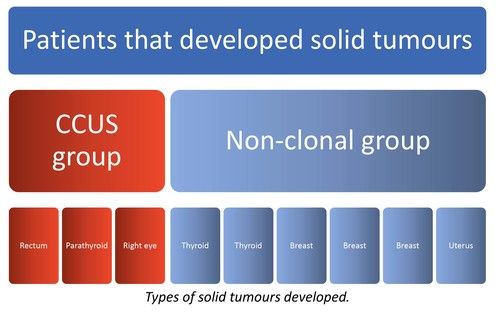
We present data from 200 adult CIN patients, 159 females and 41 males [Figure 2] (79.5% and 20.5%, respectively), aged 19-90 years [Figure 3] (median 60 years), registered from one center that accepts patients from all over Greece. Seven patients were non-Greek white Europeans and 193 were Greeks. The median time from the initial identification of neutropenia until last follow-up was 179 months (range 12-420 months) and the median time of follow-up was 133 months (range 12-396 months). The patients had mean ANCs 1370±420/μL (median 1600, range 200-1700/μL), lymphocytes 1640±475/μL (median 1600, range 700-3700/μL), monocytes 336±134/μL (median 300, range 100-800/μL), platelets 221±48 x103/μl (median 219, range 150-336 x103/μl) [Figure 4], haemoglobin 13.3±0.99 g/dL (median 13.25, range 10.6-16.5 g/dL) and MCV 88.15±5.76 fl (median 89, range 63.4-98.1 fl). Clonal hemopoiesis in NGS analysis was identified in 40 patients (20%) and these patients were further characterized as cases with clonal cytopenia of undetermined significance (CCUS). The most frequently mutated genes were DNMT3A and TET2 involving more than 50% of CCUS patients followed by BCOR/BCORL1, IDH1/2, ASXL1, SRSF2, TP53, WT1 [Figure 5][Figure 6]. Five patients (2.5%) were homozygous for the DARC/ACKR1 SNP rs2814778. Overall, five patients transformed into MDS/AML, four from the CCUS and one from the non-clonal group (P<0.0001) with a relative risk 20 for transformation in the presence of clonal disease [Figure 7]. Nine patients developed a non-hematologic malignancy, three from the CCUS and six from the non-clonal group (non-significant difference) [Figure 8]. The patients had rare infections and only one patient presented a mild covid-19 infection so far.
We present data from 200 adult CIN patients, 159 females and 41 males [Figure 2] (79.5% and 20.5%, respectively), aged 19-90 years [Figure 3] (median 60 years), registered from one center that accepts patients from all over Greece. Seven patients were non-Greek white Europeans and 193 were Greeks. The median time from the initial identification of neutropenia until last follow-up was 179 months (range 12-420 months) and the median time of follow-up was 133 months (range 12-396 months). The patients had mean ANCs 1370±420/μL (median 1600, range 200-1700/μL), lymphocytes 1640±475/μL (median 1600, range 700-3700/μL), monocytes 336±134/μL (median 300, range 100-800/μL), platelets 221±48 x103/μl (median 219, range 150-336 x103/μl) [Figure 4], haemoglobin 13.3±0.99 g/dL (median 13.25, range 10.6-16.5 g/dL) and MCV 88.15±5.76 fl (median 89, range 63.4-98.1 fl). Clonal hemopoiesis in NGS analysis was identified in 40 patients (20%) and these patients were further characterized as cases with clonal cytopenia of undetermined significance (CCUS). The most frequently mutated genes were DNMT3A and TET2 involving more than 50% of CCUS patients followed by BCOR/BCORL1, IDH1/2, ASXL1, SRSF2, TP53, WT1 [Figure 5][Figure 6]. Five patients (2.5%) were homozygous for the DARC/ACKR1 SNP rs2814778. Overall, five patients transformed into MDS/AML, four from the CCUS and one from the non-clonal group (P<0.0001) with a relative risk 20 for transformation in the presence of clonal disease [Figure 7]. Nine patients developed a non-hematologic malignancy, three from the CCUS and six from the non-clonal group (non-significant difference) [Figure 8]. The patients had rare infections and only one patient presented a mild covid-19 infection so far.
Figure 3
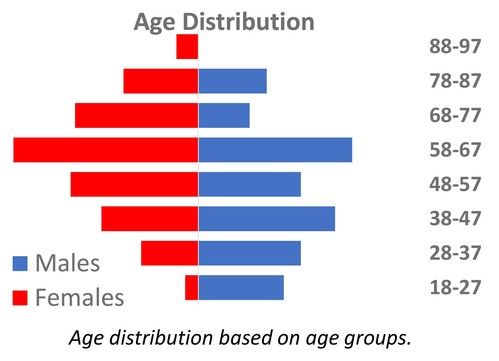
Figure 4
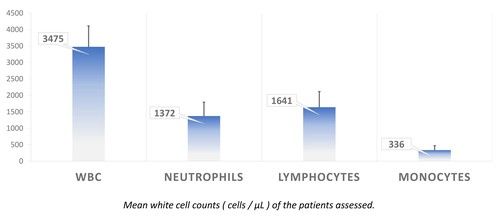
Figure 5
CONCLUSION
This is the first study presenting longitudinal clinical/laboratory data and disease outcome in CIN. The coordinated collection of common data from CIN patients from different backgrounds under EuNet-INNOCHRON is anticipated to enrich further the knowledge on the natural history of this rare disease.
This is the first study presenting longitudinal clinical/laboratory data and disease outcome in CIN. The coordinated collection of common data from CIN patients from different backgrounds under EuNet-INNOCHRON is anticipated to enrich further the knowledge on the natural history of this rare disease.
REFERENCES
- Papadaki Helen, et al. Impaired granulocytopoiesis in patients with chronic idiopathic neutropenia is associated with increased apoptosis of bone marrow myeloid progenitor cells. Blood. 2003;101(7):2591‐2600.
- Malcovati Luca, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371‐3378.
- Fragiadaki Irene, Papadakis Stavros, et al. Increased frequency of the single nucleotide polymorphism of the DARC / ACKR1 gene associated with ethnic neutropenia in a cohort of European patients with chronic idiopathic neutropenia. American Journal of Hematology. 2020;95(7):E163-E166.
Figure 6
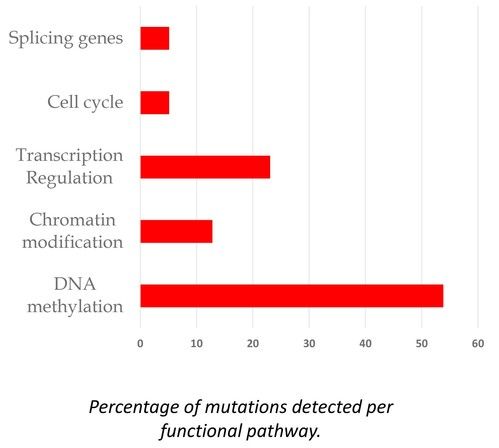
Figure 7
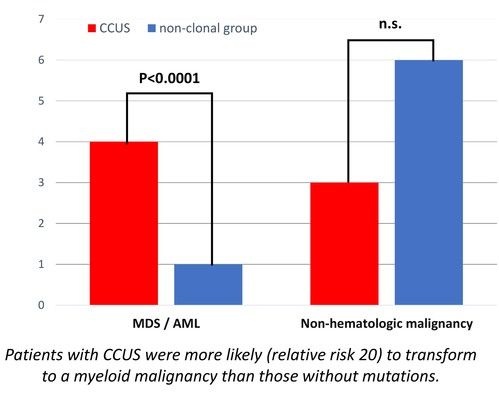
Figure 8
This article is based upon work from the European Cooperation in Science and Technology (COST) Action CA18233 “European Network for Innovative Diagnosis and treatment of Chronic Neutropenias, EuNet-INNOCHRON”
The data in this poster was presented at EHA 2021. Published with permission from the Copyright owner.
