1Department of pediatrics, College of Medicine, University of Baghdad / Hemato-Oncology Unit, Children Welfare Teaching Hospital, Medical City.
INTRODUCTION
Infections in pediatric cancer patients are the major reasons for morbidity and mortality. In cases of malignant diseases, fever may be the only apparent feature which may herald an upcoming life-threatening situation. This is particular in cases of neutropenia associated with the malignant diseases. Febrile episodes occur in about 30% of neutropenic episodes in children with chemotherapy-induced neutropenia. The approximate rate of occurrence is 0.76 episodes per every 30 days of neutropenia. [1]
The marked reduction in morbidity and mortality caused by infections among cancer children is principally related to the early and empiric use of broad-spectrum antibacterial agents during the episodes of neutropenic fever. This represented a major advance in the oncology field since the seventies of the last century.
Infections in pediatric cancer patients are the major reasons for morbidity and mortality. In cases of malignant diseases, fever may be the only apparent feature which may herald an upcoming life-threatening situation. This is particular in cases of neutropenia associated with the malignant diseases. Febrile episodes occur in about 30% of neutropenic episodes in children with chemotherapy-induced neutropenia. The approximate rate of occurrence is 0.76 episodes per every 30 days of neutropenia. [1]
The marked reduction in morbidity and mortality caused by infections among cancer children is principally related to the early and empiric use of broad-spectrum antibacterial agents during the episodes of neutropenic fever. This represented a major advance in the oncology field since the seventies of the last century.
MATERIALS AND METHODS
Type of the study: Prospective observational non-interventional study.
Study design and population: All consecutive pediatric inpatients affected by haematological diseases or solid tumours who were admitted at the pediatric oncology unit in Children Welfare Teaching Hospital (CWTH) / Medical City / Baghdad with fever and neutropenia (PMN or ANC equal or less than 500/cmm) or developed fever and neutropenia during hospitalization were enrolled into the study until hospital discharge or death.
Study duration: From September 1st 2019 to February 29th 2020 inclusive (6 months).
Ethical approval: Approved by the local ethics committee at CWTH.
Risk and benefits assessment: As this is an observational study, there are no physical potential risks or benefits to research subjects.
Forms and procedures for collecting data: The researcher was the main responsible person for filling the registry forms. There were two forms for each patient; an initial registration form and neutropenic episode form. The initial registry form included initial patient’s data encompassing demography and the initial clinical data. The neutropenic episode form adopted all the details of the episode from the start of fever until hospital discharge or death (end-point). All investigations requested and carried out, and treatment supplied were recorded in the neutropenic form. Computerized and manual consistency checks were done by meeting the supervisor frequently.
Definitions: The following definitions are not hard-and-fast rules. Clinical variations among patients necessitate that clinical judgment have a critical impact in identifying which patients require antibiotics during the risk period of neutropenia, even if those patients do not meet these specific definitions.
Infection surveillance and management: The screen for infection and management of febrile neutropenia episodes were based on local available measures and therapies and there were no special guidelines in the workup or management apart from the worldwide available knowledge that fits the CWTH policies. Blood culture were intentionally ordered by the principal investigator for all enrolled patients with few exceptions, other tissue cultures were ordered according to clinical indications. Antibacterial and antifungal therapies were prescribed as per availability.
Statistical considerations:
Sample size: The total number of patients admitted to the pediatric oncology unit was 207 patients, with a total of 350 neutropenic episodes.
Analysis: Analysis of initial data was limited to the patients (subjects). Further analysis of the data was bound to the neutropenic episodes. All eligible patients admitted during the proposed period were included in this study. Patients’ characteristics were summarized and processed by means of cross-tabulations for categorical variables or by means of independent sample T-test for continuous variables.
Program: Statistical analysis was performed with SPSS statistical program for Mac (SPSS, Chicago, IL, USA). [6]
Type of the study: Prospective observational non-interventional study.
Study design and population: All consecutive pediatric inpatients affected by haematological diseases or solid tumours who were admitted at the pediatric oncology unit in Children Welfare Teaching Hospital (CWTH) / Medical City / Baghdad with fever and neutropenia (PMN or ANC equal or less than 500/cmm) or developed fever and neutropenia during hospitalization were enrolled into the study until hospital discharge or death.
Study duration: From September 1st 2019 to February 29th 2020 inclusive (6 months).
Ethical approval: Approved by the local ethics committee at CWTH.
Risk and benefits assessment: As this is an observational study, there are no physical potential risks or benefits to research subjects.
Forms and procedures for collecting data: The researcher was the main responsible person for filling the registry forms. There were two forms for each patient; an initial registration form and neutropenic episode form. The initial registry form included initial patient’s data encompassing demography and the initial clinical data. The neutropenic episode form adopted all the details of the episode from the start of fever until hospital discharge or death (end-point). All investigations requested and carried out, and treatment supplied were recorded in the neutropenic form. Computerized and manual consistency checks were done by meeting the supervisor frequently.
Definitions: The following definitions are not hard-and-fast rules. Clinical variations among patients necessitate that clinical judgment have a critical impact in identifying which patients require antibiotics during the risk period of neutropenia, even if those patients do not meet these specific definitions.
- Febrile neutropenia: A single oral temperature 38.3°C or an oral temperature 38.0°C sustained for 1 hours or that occurs twice within a 24-h period with an ANC < 0.5 * 109 /l or ANC < 1.0 * 109/l expected to decrease to < 0.5 * 109 /l over the subsequent 48 hours. [2]
- Microbiologically documented infections: An infection that was clinically detectable and microbiologically proven. [3]
- Clinically documented infection: A site of infection that is diagnosed but its microbiological pathogenesis either cannot be proven. [3]
- End of neutropenic episode: For the purpose of the study, this was defined as the time of death or discharge of patient after stopping parenteral antibiotic therapy and being afebrile for 48 hours.
- Performance status / score: The Eastern Cooperative Oncology Group(ECOG) Scale of Performance Status is one such measurement. It describes a patient’s level of functioning in terms of their ability to care for themself, daily activity, and physical ability (walking, working, etc.). [4]
Infection surveillance and management: The screen for infection and management of febrile neutropenia episodes were based on local available measures and therapies and there were no special guidelines in the workup or management apart from the worldwide available knowledge that fits the CWTH policies. Blood culture were intentionally ordered by the principal investigator for all enrolled patients with few exceptions, other tissue cultures were ordered according to clinical indications. Antibacterial and antifungal therapies were prescribed as per availability.
Statistical considerations:
Sample size: The total number of patients admitted to the pediatric oncology unit was 207 patients, with a total of 350 neutropenic episodes.
Analysis: Analysis of initial data was limited to the patients (subjects). Further analysis of the data was bound to the neutropenic episodes. All eligible patients admitted during the proposed period were included in this study. Patients’ characteristics were summarized and processed by means of cross-tabulations for categorical variables or by means of independent sample T-test for continuous variables.
Program: Statistical analysis was performed with SPSS statistical program for Mac (SPSS, Chicago, IL, USA). [6]
Figure 1
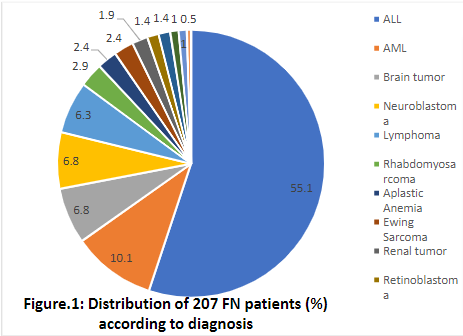
Table 1
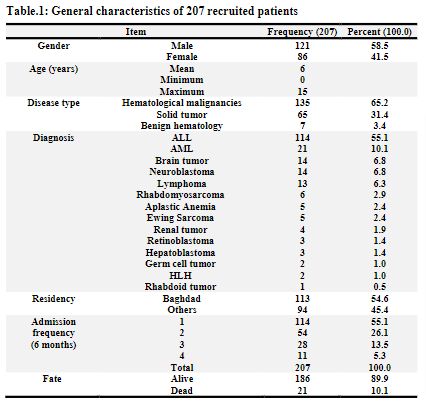
Table 4
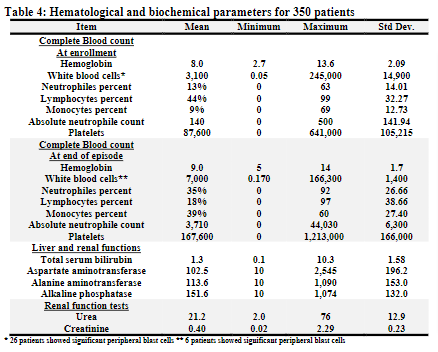
Table 5
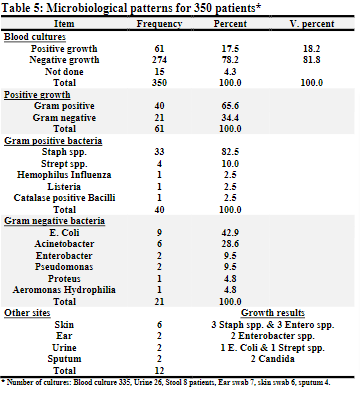
Table 7
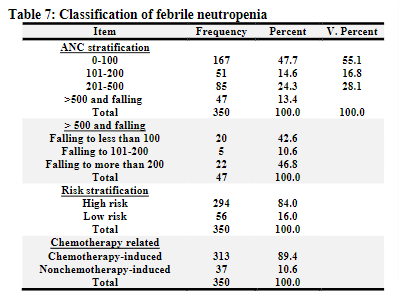
Table 8
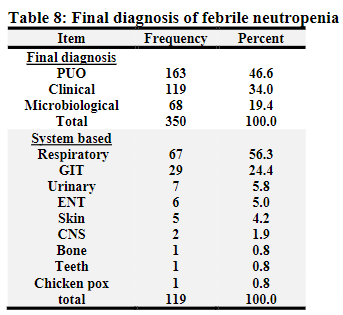
Table 12
RESULTS AND DISCUSSION
Patients:
A total of 350 febrile neutropenic episodes among pediatric patients with hematological conditions or cancer, occurring in 207 patients, were registered. All those patients were admitted and treated in hematology oncology unit in CWTH, medical city, Baghdad.The accrual of patients started on 1stSeptember 2019 and ended on 29thFebruary 2020 (six months). The follow up for each patient ended upon discharge or death.The majority of patients are with hematological malignancies (65.2%). ALL was the most frequently reported underlying malignancy in 114 (55.1%) patients.The mean age was 6 years, males being 121 patients (58.5%). Baghdad was the major source of cases in more than half of patients. More than half of the patients were admitted once during the study period. Forty-four percent of patients reported more than one episode of febrile neutropenia that required admission to the hospital.The age and gender distribution kept in pace with other studies. [7]
Febrile neutropenia episodes:
The following analysis was confined to the FN episodes and each episode was referred to as (patient/episode/case):
Diagnosis:
The majority of reported underlying diagnoses belong to hematological malignancies as they were reported in 236 patients (67.4%). Of these total episodes, ALL was reported to be the most frequent malady in 197 episodes (56.3%), followed by AML in 39 episodes (11.1%), then neuroblastoma (6.3%), brain tumors (5.1%), and lymphomas and Rhabdomyosarcoma (4.9% for each).In the current study, hematological malignancies were found to be the commonest underlying associated type of disease. Bone marrow involvement, intensity of therapy, long duration of treatment, and impaired phagocytosis and killing of pathogens (functional neutropenia) are possible facts making these diseases at high risk for infections. Additionally, the use of prophylactic Neopogen in many solid tumors may play a rule in this finding.
Disease phase and risk group:
Among leukemia patients, twenty-one percent of the episodes were registered atthe onset of the disease (at presentation or after first treatment). The majority of episodes were recruited during complete remission of the disease (61.9%). Out of the 197 ALL FN episodes registered in the study, one third of cases were stratified to beon group B protocol.
For ALL, AML, and LL cases, those during maintenance courses of their treatment constitutes the majority of cases, reported in 90 episodes (37.2%). This was followed by consolidation/Intensification phase of treatment reported in 74 patients (30.6%).
Comorbidities:
Around 25% of cases were having comorbidities which were recognized as following: 39 patients with prior therapy for cancer (relapsed, progressive diseases, or radiotherapy); 24 patients with a variable degree of liver impairment due to hepatitis C virus infection; 6 patients with cardiac problems (congenital or acquired); 6 patients with CNS morbidities (Shunt, CP, or epilepsy); 4 patients with single kidney (Nephrectomy); 3 patients with genetic syndromes (Down and Neurofibromatosis); 2 patients with respiratory morbidities; and 2 patients with GIT problems.
Complaints and clinical characteristics at time of enrollment:
Around forty percent of patients recalled or had shown respiratory symptoms. Abdominal complaints were reported in 178 patients (50.9%).Bleeding was recorded in 79 patients (22.6%).Mucositis was noted in 187 patients (53.4%), grade II being the most frequently notified in 83 patients (44.4%), followed by grade I in 74 patients (39.6%), grade III in 26 patients (13.9%) and grade IV in 4 patients (2.1%). Performance score according to ECOG was assessed in those patients; score IV was noted in 13 patients (3.8%) of the whole group while the majority had shown a score of II (48.4%).
Hematological and biochemical parameters:
At time of enrollment: The mean hemoglobin level for this cohort was 8.0 gm/dl, the mean WBC count was 3,100 /cmm, the mean platelets level was 87,600 /cmm, and the mean ANC was 140 with a range of (0 –500).
At time of end of the episode: The mean hemoglobin level was 9.0 gm/dl, the mean WBC count was 7,000 /cmm, the mean platelet level was 167,600 /cmm, and the mean ANC was 3,710 (range 0 –44,030).
Microbiological studies:
Blood culture: Blood was withdrawn for culture and sensitivity at time of admission for most patients with exception of few patients whom blood culture was ordered for them after one or two days of admission and starting antibiotic therapy. Fifteen patients failed to withdraw samples for blood culture. Blood culture was ordered by the principal investigator.
Blood growth results: Sixty-one episodes showed positive bacterial growth (18.2%), while 274 showed no growth (81.8%). The growth showed gram-positive bacteria in two-third of episodes (65.6%), while gram-negative was shown in one-third (34.4%). Of the 40 patients who showed gram-positive bacterial growth, Staphylococcal species was reported in 33 samples (82.5%), followed by Streptococcal growth in 10%. Escherichia Coli was grown in (42.9%) of the shown gram-negative bacterial growth, followed by Acinetobacter species in 6 growths (28.6%).The frequency of pathogenic organisms varies from institution to institution. In general, there is a global shift toward a dominance of gram-positive organisms due to the ubiquitous use of prophylactic antimicrobials and indwelling venous catheters. The current study shows that gram positive pathogens are the most frequent, coagulase-negative species being the most common. Anywise, there is no routine use of indwelling venous catheters in the studied center. This result is similar to other literatures. [1,8,9]
Aerobic gram-negative bacilli accounted for approximately one-third of bacteremic episodes, with Escherichia coli, Acinetobacterspp, andEnterobacterspp were among the commonest isolates. Again, this agrees with other literatures. [1,8,9]
Other sites: Urine was sent for culture in 26 patients (2 positive), stool in 8 patients (all negative), Ear swab in 7 patients (2 positive),skin lesion swab in 7 patients (6 positive), and sputum in 4 patients (2 positive). Culture and sensitivity swabs/samples were ordered according to the opinion of treating physician. Skin lesions showed 3 staph spp. growths and 3 Enterobacter spp. growth. Sputum growth showed Candida growth in both samples. From the 61 positive blood cultures, five were positive for other cultures also (Combined growth: 4 skin and 1 ear).Two sputum samples were positive for candida growth. In recent years, both animal experimentation and human observations have provided provocative evidence that Candida spp. may represent more than an innocent bystander, both in infectious and non-infectious disease states. The clinical significance of the detection of Candida spp. in the respiratory tract is increasingly uncertain and yet to be spoken. [10]
Growth Sensitivity: Of the 61 positive bacterial growth detected, Gentamicin had shown to be the most frequently reported antibiotic that showed positive sensitivity in about half of the cases. One-third shown sensitivity to Amikacin, Meropenem and Imipenem. Those are followed in order of frequency by Ciprofloxacin (27.9%), Ampicillin (23.0%), Azithromycin (21.3%), and Piperacillin / tazobactam (19.7%).
Classification of FN episodes:
ANC-based classification: According to the level of ANC at time of enrollment, profound neutropenia (ANC equal or less than 100) was the most frequently encountered class in 167 patients (55.1%). Forty-seven patients (13.4%) presented with ANC more than 500, which later fell to less than 500. Of them, the major proportion (46.8%) fell to a level between (201-500).
Risk stratification: The majority of patients (84.0%) belong to high-risk FN according to the level of ANC, type of disease, chemotherapy phase, clinical signs and symptoms. Fifty-six patients (16.0%) were low-risk patients.
Chemotherapy-induced FN: Out of the whole cohort, 313 patients were admitted with Chemotherapy-Induced FN (89.4%). Thirty-seven patients (10.6%) were registered as Nonchemotherapy-Induced FN. This cohort showed that 84% of cases were lying under the category of high-risk FN. The differentiation was based upon presenting signs and symptoms, counts, underlying cancer, type of therapy, the anticipated length of neutropenia, and associated medical conditions. However, there is no currently agreed-upon risk stratification specifically for children as most of the written documents were based on extrapolation of adult guidelines. Randomized controlled trials to determine the appropriate settings in pediatric FN are lacking. Yet, most of the reports stated that carefully selected low-risk patients may be candidates for oral empiric therapy or outpatient treatment. [11]
Final diagnoses:
The final diagnoses were assessed and tabulated after review of the complaints, presentation, examination notes, result of investigations and course of disease during hospitalization. Out of the recruited group, 163 patients were labelled as fever without origin as the final diagnosis (46.4%). This was followed by a group of 119 patients (34.0%) with a presumptive diagnosis of “clinical infection” (according to the above-mentioned data). Microbiological diagnoses were reported in 68 patients; 61 blood, 2 skin, 2 sputum, 2 urine, and 1 ear. Clinical diagnoses were reported in 119 patients; of them the respiratory-based diagnosis was the most frequently identified in 67 patients (56.3%). This included upper and lower respiratory tract infections. This was followed by GIT problems, the majority being gastritis or gastroenteritis. Urinary tract infections were postulated to be found in 7 patients, the majority complained from dysuria, despite only two positive cultures for urine. Regarding the diagnosis of FN; the rate of clinically documented infection, when a child presents with fever and therapy-induced neutropenia, ranges between 10 and 40 percent in many literatures. In this study, it was shown that clinically documented infections compromise 34% of the sample size. [1,8,9]
Treatment of FN episodes:
The majority of patients admitted for treatment of FN in CWTH started on two drug therapies, others started as per clinical condition and opinion of treating physician. Seventy-five percent of patients started two drug therapies. This regimen included (173 Tazocin & Amikacin; 52 Ceftriaxone & Amikacin; 37 Meropenem & Amikacin; and 3 others)Fifty-nine patients (16.8%) were started on three drug therapies. Therapy was consisted of (12 Ceftriaxone, Amikacin and Metronidazole; 12 Tazocin, Amikacin and Metronidazole; 11 Tazocin, Amikacin and Fluconazole; 5 Meropenem, Amikacin and Metronidazole; 4 Meropenem, Amikacin and Fluconazole; 3 Meropenem, Amikacin and Azithromycin; and 12 others)
Final Destiney: Out of the cohort group, 21 patients (6.0%) died as a complication of FN. Thirteen patients (61.9%) died with septic shock. Four patients (19.0%) died with a presumptive diagnosis of fungal pneumonia. One patient died suddenly with a possible / presumptive diagnosis of intracranial bleeding; another patient died with a possible / presumptive diagnosis of graft-versus-host disease after receiving blood transfusion. One patient died suddenly after admission due to FN and a features of chronic liver disease. One patient died with a proven brain abscess. Of the deceased patients, sixteen patients (76.2%) were reported with a diagnosis of hematological malignancies (10 ALL & 6 AML). Four patients died within 24 hours of admission. The infection-related mortality in this study was 6%. This was considered as large figure as compared with other data. The mortality usually is different from center to other depending on the institution, facilities, infrastructure and availability of supportive care. The mortality in different centers is ranging from (0.5-6.6%). [8, 12-15]
Patients:
A total of 350 febrile neutropenic episodes among pediatric patients with hematological conditions or cancer, occurring in 207 patients, were registered. All those patients were admitted and treated in hematology oncology unit in CWTH, medical city, Baghdad.The accrual of patients started on 1stSeptember 2019 and ended on 29thFebruary 2020 (six months). The follow up for each patient ended upon discharge or death.The majority of patients are with hematological malignancies (65.2%). ALL was the most frequently reported underlying malignancy in 114 (55.1%) patients.The mean age was 6 years, males being 121 patients (58.5%). Baghdad was the major source of cases in more than half of patients. More than half of the patients were admitted once during the study period. Forty-four percent of patients reported more than one episode of febrile neutropenia that required admission to the hospital.The age and gender distribution kept in pace with other studies. [7]
Febrile neutropenia episodes:
The following analysis was confined to the FN episodes and each episode was referred to as (patient/episode/case):
Diagnosis:
The majority of reported underlying diagnoses belong to hematological malignancies as they were reported in 236 patients (67.4%). Of these total episodes, ALL was reported to be the most frequent malady in 197 episodes (56.3%), followed by AML in 39 episodes (11.1%), then neuroblastoma (6.3%), brain tumors (5.1%), and lymphomas and Rhabdomyosarcoma (4.9% for each).In the current study, hematological malignancies were found to be the commonest underlying associated type of disease. Bone marrow involvement, intensity of therapy, long duration of treatment, and impaired phagocytosis and killing of pathogens (functional neutropenia) are possible facts making these diseases at high risk for infections. Additionally, the use of prophylactic Neopogen in many solid tumors may play a rule in this finding.
Disease phase and risk group:
Among leukemia patients, twenty-one percent of the episodes were registered atthe onset of the disease (at presentation or after first treatment). The majority of episodes were recruited during complete remission of the disease (61.9%). Out of the 197 ALL FN episodes registered in the study, one third of cases were stratified to beon group B protocol.
For ALL, AML, and LL cases, those during maintenance courses of their treatment constitutes the majority of cases, reported in 90 episodes (37.2%). This was followed by consolidation/Intensification phase of treatment reported in 74 patients (30.6%).
Comorbidities:
Around 25% of cases were having comorbidities which were recognized as following: 39 patients with prior therapy for cancer (relapsed, progressive diseases, or radiotherapy); 24 patients with a variable degree of liver impairment due to hepatitis C virus infection; 6 patients with cardiac problems (congenital or acquired); 6 patients with CNS morbidities (Shunt, CP, or epilepsy); 4 patients with single kidney (Nephrectomy); 3 patients with genetic syndromes (Down and Neurofibromatosis); 2 patients with respiratory morbidities; and 2 patients with GIT problems.
Complaints and clinical characteristics at time of enrollment:
Around forty percent of patients recalled or had shown respiratory symptoms. Abdominal complaints were reported in 178 patients (50.9%).Bleeding was recorded in 79 patients (22.6%).Mucositis was noted in 187 patients (53.4%), grade II being the most frequently notified in 83 patients (44.4%), followed by grade I in 74 patients (39.6%), grade III in 26 patients (13.9%) and grade IV in 4 patients (2.1%). Performance score according to ECOG was assessed in those patients; score IV was noted in 13 patients (3.8%) of the whole group while the majority had shown a score of II (48.4%).
Hematological and biochemical parameters:
At time of enrollment: The mean hemoglobin level for this cohort was 8.0 gm/dl, the mean WBC count was 3,100 /cmm, the mean platelets level was 87,600 /cmm, and the mean ANC was 140 with a range of (0 –500).
At time of end of the episode: The mean hemoglobin level was 9.0 gm/dl, the mean WBC count was 7,000 /cmm, the mean platelet level was 167,600 /cmm, and the mean ANC was 3,710 (range 0 –44,030).
Microbiological studies:
Blood culture: Blood was withdrawn for culture and sensitivity at time of admission for most patients with exception of few patients whom blood culture was ordered for them after one or two days of admission and starting antibiotic therapy. Fifteen patients failed to withdraw samples for blood culture. Blood culture was ordered by the principal investigator.
Blood growth results: Sixty-one episodes showed positive bacterial growth (18.2%), while 274 showed no growth (81.8%). The growth showed gram-positive bacteria in two-third of episodes (65.6%), while gram-negative was shown in one-third (34.4%). Of the 40 patients who showed gram-positive bacterial growth, Staphylococcal species was reported in 33 samples (82.5%), followed by Streptococcal growth in 10%. Escherichia Coli was grown in (42.9%) of the shown gram-negative bacterial growth, followed by Acinetobacter species in 6 growths (28.6%).The frequency of pathogenic organisms varies from institution to institution. In general, there is a global shift toward a dominance of gram-positive organisms due to the ubiquitous use of prophylactic antimicrobials and indwelling venous catheters. The current study shows that gram positive pathogens are the most frequent, coagulase-negative species being the most common. Anywise, there is no routine use of indwelling venous catheters in the studied center. This result is similar to other literatures. [1,8,9]
Aerobic gram-negative bacilli accounted for approximately one-third of bacteremic episodes, with Escherichia coli, Acinetobacterspp, andEnterobacterspp were among the commonest isolates. Again, this agrees with other literatures. [1,8,9]
Other sites: Urine was sent for culture in 26 patients (2 positive), stool in 8 patients (all negative), Ear swab in 7 patients (2 positive),skin lesion swab in 7 patients (6 positive), and sputum in 4 patients (2 positive). Culture and sensitivity swabs/samples were ordered according to the opinion of treating physician. Skin lesions showed 3 staph spp. growths and 3 Enterobacter spp. growth. Sputum growth showed Candida growth in both samples. From the 61 positive blood cultures, five were positive for other cultures also (Combined growth: 4 skin and 1 ear).Two sputum samples were positive for candida growth. In recent years, both animal experimentation and human observations have provided provocative evidence that Candida spp. may represent more than an innocent bystander, both in infectious and non-infectious disease states. The clinical significance of the detection of Candida spp. in the respiratory tract is increasingly uncertain and yet to be spoken. [10]
Growth Sensitivity: Of the 61 positive bacterial growth detected, Gentamicin had shown to be the most frequently reported antibiotic that showed positive sensitivity in about half of the cases. One-third shown sensitivity to Amikacin, Meropenem and Imipenem. Those are followed in order of frequency by Ciprofloxacin (27.9%), Ampicillin (23.0%), Azithromycin (21.3%), and Piperacillin / tazobactam (19.7%).
Classification of FN episodes:
ANC-based classification: According to the level of ANC at time of enrollment, profound neutropenia (ANC equal or less than 100) was the most frequently encountered class in 167 patients (55.1%). Forty-seven patients (13.4%) presented with ANC more than 500, which later fell to less than 500. Of them, the major proportion (46.8%) fell to a level between (201-500).
Risk stratification: The majority of patients (84.0%) belong to high-risk FN according to the level of ANC, type of disease, chemotherapy phase, clinical signs and symptoms. Fifty-six patients (16.0%) were low-risk patients.
Chemotherapy-induced FN: Out of the whole cohort, 313 patients were admitted with Chemotherapy-Induced FN (89.4%). Thirty-seven patients (10.6%) were registered as Nonchemotherapy-Induced FN. This cohort showed that 84% of cases were lying under the category of high-risk FN. The differentiation was based upon presenting signs and symptoms, counts, underlying cancer, type of therapy, the anticipated length of neutropenia, and associated medical conditions. However, there is no currently agreed-upon risk stratification specifically for children as most of the written documents were based on extrapolation of adult guidelines. Randomized controlled trials to determine the appropriate settings in pediatric FN are lacking. Yet, most of the reports stated that carefully selected low-risk patients may be candidates for oral empiric therapy or outpatient treatment. [11]
Final diagnoses:
The final diagnoses were assessed and tabulated after review of the complaints, presentation, examination notes, result of investigations and course of disease during hospitalization. Out of the recruited group, 163 patients were labelled as fever without origin as the final diagnosis (46.4%). This was followed by a group of 119 patients (34.0%) with a presumptive diagnosis of “clinical infection” (according to the above-mentioned data). Microbiological diagnoses were reported in 68 patients; 61 blood, 2 skin, 2 sputum, 2 urine, and 1 ear. Clinical diagnoses were reported in 119 patients; of them the respiratory-based diagnosis was the most frequently identified in 67 patients (56.3%). This included upper and lower respiratory tract infections. This was followed by GIT problems, the majority being gastritis or gastroenteritis. Urinary tract infections were postulated to be found in 7 patients, the majority complained from dysuria, despite only two positive cultures for urine. Regarding the diagnosis of FN; the rate of clinically documented infection, when a child presents with fever and therapy-induced neutropenia, ranges between 10 and 40 percent in many literatures. In this study, it was shown that clinically documented infections compromise 34% of the sample size. [1,8,9]
Treatment of FN episodes:
The majority of patients admitted for treatment of FN in CWTH started on two drug therapies, others started as per clinical condition and opinion of treating physician. Seventy-five percent of patients started two drug therapies. This regimen included (173 Tazocin & Amikacin; 52 Ceftriaxone & Amikacin; 37 Meropenem & Amikacin; and 3 others)Fifty-nine patients (16.8%) were started on three drug therapies. Therapy was consisted of (12 Ceftriaxone, Amikacin and Metronidazole; 12 Tazocin, Amikacin and Metronidazole; 11 Tazocin, Amikacin and Fluconazole; 5 Meropenem, Amikacin and Metronidazole; 4 Meropenem, Amikacin and Fluconazole; 3 Meropenem, Amikacin and Azithromycin; and 12 others)
Final Destiney: Out of the cohort group, 21 patients (6.0%) died as a complication of FN. Thirteen patients (61.9%) died with septic shock. Four patients (19.0%) died with a presumptive diagnosis of fungal pneumonia. One patient died suddenly with a possible / presumptive diagnosis of intracranial bleeding; another patient died with a possible / presumptive diagnosis of graft-versus-host disease after receiving blood transfusion. One patient died suddenly after admission due to FN and a features of chronic liver disease. One patient died with a proven brain abscess. Of the deceased patients, sixteen patients (76.2%) were reported with a diagnosis of hematological malignancies (10 ALL & 6 AML). Four patients died within 24 hours of admission. The infection-related mortality in this study was 6%. This was considered as large figure as compared with other data. The mortality usually is different from center to other depending on the institution, facilities, infrastructure and availability of supportive care. The mortality in different centers is ranging from (0.5-6.6%). [8, 12-15]
RESULTS AND DISCUSSION (CONT)
Time and duration characteristics of the episodes:
The mean duration of chemotherapy treatment was 29.9 weeks in 316 patients. The mean duration since last treatment was 5.7 days in 313 patients. The mean neutropenia duration before fever was 6.4 days in 88 patients. The mean FN duration before admission was 3.3 days in 261 patients. The mean antibiotic use duration before admission was 3.5 days 72 patients. The mean duration of Neopogen was 5.6 days in 96 patients. The mean duration of FN stay was 7.8 days in the whole group.
Mortality perception
Continuous variables: The mean duration of fever before admission for the deceased group was 9.1 days which was significantly longer than the duration for the alive group (mean duration 3.0 days), the length of stay was 7.7 days for the alive group versus 8.8 days for the deceased group. Four patients died within the same day of admission.
Categorial variables: Correlation of different categorial variables to the outcome has shown the following results; there was no significant correlation between the outcome and each of the gender, source of referral, presence or absence of comorbidities, risk group in ALL, and the diagnosis of the patients. On the other side, there was a significant statistical correlation between the fate of the patients and the following parameters: Most of the deceased patients belong to hematological malignancies (P value 0.007). Of those ALL patients who developed FN before induction therapy; nobody died. The statistical significance showed significant correlation between induction, consolidation and intensification phases and death (P value 0.005). There was a significant correlation between number of treatment lines and survival (P value 0.008). there was a significant correlation between chemotherapy-wise neutropenia and death in this cohort group (P value 0.006). All deceased patients were having a high-risk FN (P value 0.03). Half of the deceased patients failed to grow a bacterium in their blood culture samples. The statistical correlation was significant. (0.001).Finally, the provision of antibiotic and antifungal therapy in this study was based mainly on the acquired knowledge of the treating physicians. Yet, it was hindered largely by the availability of antibiotics.
Time and duration characteristics of the episodes:
The mean duration of chemotherapy treatment was 29.9 weeks in 316 patients. The mean duration since last treatment was 5.7 days in 313 patients. The mean neutropenia duration before fever was 6.4 days in 88 patients. The mean FN duration before admission was 3.3 days in 261 patients. The mean antibiotic use duration before admission was 3.5 days 72 patients. The mean duration of Neopogen was 5.6 days in 96 patients. The mean duration of FN stay was 7.8 days in the whole group.
Mortality perception
Continuous variables: The mean duration of fever before admission for the deceased group was 9.1 days which was significantly longer than the duration for the alive group (mean duration 3.0 days), the length of stay was 7.7 days for the alive group versus 8.8 days for the deceased group. Four patients died within the same day of admission.
Categorial variables: Correlation of different categorial variables to the outcome has shown the following results; there was no significant correlation between the outcome and each of the gender, source of referral, presence or absence of comorbidities, risk group in ALL, and the diagnosis of the patients. On the other side, there was a significant statistical correlation between the fate of the patients and the following parameters: Most of the deceased patients belong to hematological malignancies (P value 0.007). Of those ALL patients who developed FN before induction therapy; nobody died. The statistical significance showed significant correlation between induction, consolidation and intensification phases and death (P value 0.005). There was a significant correlation between number of treatment lines and survival (P value 0.008). there was a significant correlation between chemotherapy-wise neutropenia and death in this cohort group (P value 0.006). All deceased patients were having a high-risk FN (P value 0.03). Half of the deceased patients failed to grow a bacterium in their blood culture samples. The statistical correlation was significant. (0.001).Finally, the provision of antibiotic and antifungal therapy in this study was based mainly on the acquired knowledge of the treating physicians. Yet, it was hindered largely by the availability of antibiotics.
CONCLUSIONS
ALL was the most frequently encountered underlying malignancy in febrile neutropenia, AML was associated with the worst outcome. The result of blood cultures was comparable to worldwide results. Gram positive organisms were the most commonly identified growth. High-risk FN was the commonest reported type, low-risk being lowest because of possible treatment outside the hospital on oral-based antibiotics. Still nearly half of the patients were showing no hints for any clinical diagnosis and presented with fever without any localizing signs. Infection related mortality is high. Provision of bacterial growth culture media is essential for better care of patients with FN. Supportive care is of paramount importance in providing a perfect outcome for patients with febrile neutropenia. Appreciation of blood culture and sensitivity results is important in treating patients with FN. Patients with low-risk FN should be treated on OP bases to lessen the burden of work on the inpatient service.
ALL was the most frequently encountered underlying malignancy in febrile neutropenia, AML was associated with the worst outcome. The result of blood cultures was comparable to worldwide results. Gram positive organisms were the most commonly identified growth. High-risk FN was the commonest reported type, low-risk being lowest because of possible treatment outside the hospital on oral-based antibiotics. Still nearly half of the patients were showing no hints for any clinical diagnosis and presented with fever without any localizing signs. Infection related mortality is high. Provision of bacterial growth culture media is essential for better care of patients with FN. Supportive care is of paramount importance in providing a perfect outcome for patients with febrile neutropenia. Appreciation of blood culture and sensitivity results is important in treating patients with FN. Patients with low-risk FN should be treated on OP bases to lessen the burden of work on the inpatient service.
REFERENCES
1. Castagnola E, Fontana V, Caviglia I, et al, A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation, Clin Infect Dis. 2007;45(10):1296.
2. Agrawal K. and James F. Supportive Care of Patients with Cancer, in [Manual of pediatric hematology and oncology], Lanzkowsky P (Eds). 6th ed., Elsevier Academic press; 2016. p. 620-655.
3. Haeusler GM, Phillips RS, Lehrnbecher T, Thursky KA, Sung L, Ammann RA. Core outcomes and definitions for pediatric fever and neutropenia research: a consensus statement from an international panel. Pediatr Blood Cancer 2015;62:483–9.
4. Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group.Am J Clin Oncol.1982;5: 649-655.
5. Hastings C, Torkildson J, Agrawal K. Management of Fever in the Child with Cancer, in [Handbook of Pediatric Hematology and Oncology], Hastings C (Eds). 2nd ed., Wiley-Blackwell; 2012. p. 244-255.
6. Nourusis MJ. APSS statistical software. SPSS: Base and Advanced statistics 20.0. Chicago, SPSS Inc, 2014.
7. Gabrielle M, Karin A, Monica A, et al. Risk stratification in children with cancer and febrile neutropenia: A national, prospective, multicentre validation of nine clinical decision rules. Eclinical Medicine 2020;18, 100220, 1-9.
8. Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH, Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31(9):623.
9. Agyeman P, Aebi C, Hirt A, et al. Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Infect Dis J 2011; 30:e114.
10. Kathryn M, Gary B, and Robert P. The significance of Candida in the human respiratory tract: our evolving understanding Pathogens and Disease, 2017; Vol. 75, No. 3, P 1-6.
11. Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J Clin Oncol 2017; 35:2082.
12. Ariffin H, Navaratnam P, Lin HP. Surveillance study of bacteraemic episodes in febrile neutropenic children. Int J Clin Pract. 2002;56:237–240.
13. Lehrnbecher T, Varwig D, Kaiser J, et al. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia.2004;18:72–77.
14. Santolaya ME, Alvarez AM, Aviles CL, et al. Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatr Infect Dis J. 2007;26:794–798.
15. Klaassen RJ, Goodman TR, PhamB, et al. ‘‘Low-risk’’ prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000;18:1012–1019.
1. Castagnola E, Fontana V, Caviglia I, et al, A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation, Clin Infect Dis. 2007;45(10):1296.
2. Agrawal K. and James F. Supportive Care of Patients with Cancer, in [Manual of pediatric hematology and oncology], Lanzkowsky P (Eds). 6th ed., Elsevier Academic press; 2016. p. 620-655.
3. Haeusler GM, Phillips RS, Lehrnbecher T, Thursky KA, Sung L, Ammann RA. Core outcomes and definitions for pediatric fever and neutropenia research: a consensus statement from an international panel. Pediatr Blood Cancer 2015;62:483–9.
4. Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group.Am J Clin Oncol.1982;5: 649-655.
5. Hastings C, Torkildson J, Agrawal K. Management of Fever in the Child with Cancer, in [Handbook of Pediatric Hematology and Oncology], Hastings C (Eds). 2nd ed., Wiley-Blackwell; 2012. p. 244-255.
6. Nourusis MJ. APSS statistical software. SPSS: Base and Advanced statistics 20.0. Chicago, SPSS Inc, 2014.
7. Gabrielle M, Karin A, Monica A, et al. Risk stratification in children with cancer and febrile neutropenia: A national, prospective, multicentre validation of nine clinical decision rules. Eclinical Medicine 2020;18, 100220, 1-9.
8. Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH, Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31(9):623.
9. Agyeman P, Aebi C, Hirt A, et al. Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Infect Dis J 2011; 30:e114.
10. Kathryn M, Gary B, and Robert P. The significance of Candida in the human respiratory tract: our evolving understanding Pathogens and Disease, 2017; Vol. 75, No. 3, P 1-6.
11. Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J Clin Oncol 2017; 35:2082.
12. Ariffin H, Navaratnam P, Lin HP. Surveillance study of bacteraemic episodes in febrile neutropenic children. Int J Clin Pract. 2002;56:237–240.
13. Lehrnbecher T, Varwig D, Kaiser J, et al. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia.2004;18:72–77.
14. Santolaya ME, Alvarez AM, Aviles CL, et al. Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatr Infect Dis J. 2007;26:794–798.
15. Klaassen RJ, Goodman TR, PhamB, et al. ‘‘Low-risk’’ prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000;18:1012–1019.
The data in this poster was presented at EHA 2021. Published with permission from the Copyright owner.
