1Houston Methodist Cancer Center, Houston TX,
BACKGROUND
Pancreatic adenocarcinoma (PDAC) is one of the most aggressive cancer histologies, with high recurrence (85%) and a 5-year survival rate of 9% (1,2). Numerous circulating biomarkers have been evaluated for the detection of molecular residual disease (MRD) and molecular monitoring in PDAC, however, the application of these techniques has been limited in early-stage PDAC due to poor sensitivity and specificity (3). Recently, an ultrasensitive, personalized, and tumor-informed ctDNA assay (SignateraTM) has shown to overcome many of the challenges that have plagued the aforementioned biomarkers, allowing for reliable detection of MRD across different tumors.
Pancreatic adenocarcinoma (PDAC) is one of the most aggressive cancer histologies, with high recurrence (85%) and a 5-year survival rate of 9% (1,2). Numerous circulating biomarkers have been evaluated for the detection of molecular residual disease (MRD) and molecular monitoring in PDAC, however, the application of these techniques has been limited in early-stage PDAC due to poor sensitivity and specificity (3). Recently, an ultrasensitive, personalized, and tumor-informed ctDNA assay (SignateraTM) has shown to overcome many of the challenges that have plagued the aforementioned biomarkers, allowing for reliable detection of MRD across different tumors.
METHODS
In this cohort, 7 patients with pancreatic cancer and 1 patient with ampullary adenocarcinoma were prospectively enrolled for ctDNA analysis and followed up for a median of 316days (range: 152-684). Personalized mutational profiles derived from tumor tissue via whole-exome sequencing were used to design patient-specific ctDNA assays for variant detection in plasma samples. Apart from ctDNA analysis, patients were also monitoredusing carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA 19-9), and radiological imaging.
In this cohort, 7 patients with pancreatic cancer and 1 patient with ampullary adenocarcinoma were prospectively enrolled for ctDNA analysis and followed up for a median of 316days (range: 152-684). Personalized mutational profiles derived from tumor tissue via whole-exome sequencing were used to design patient-specific ctDNA assays for variant detection in plasma samples. Apart from ctDNA analysis, patients were also monitoredusing carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA 19-9), and radiological imaging.
RESULTS
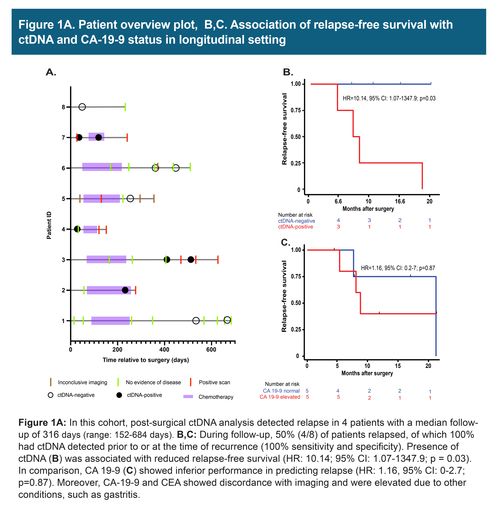
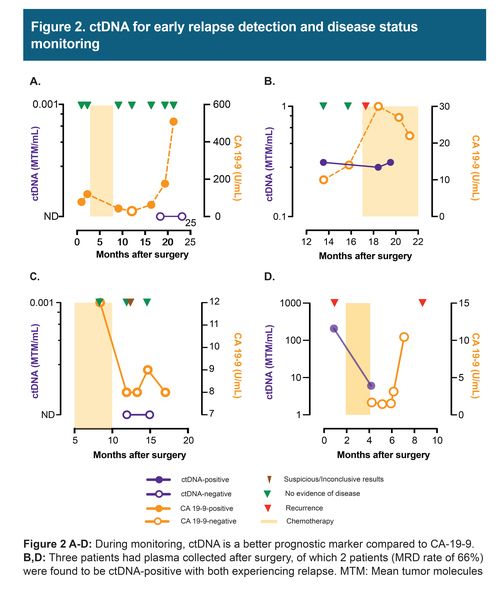
In these 8 cases of resectable pancreatic/ampullary adenocarcinoma, 7 patients received mFOLFIRINOX adjuvant chemotherapy and 2 received additional rounds of gemcitabine/oxaliplatin and gemcitabine/paclitaxel. Three patients had plasma collected after surgery, of which 2 patients were found to be ctDNA-positive and eventually relapsed. During the course of the follow-up, 50% (4/8) of patients relapsed. The presence of ctDNA was associated with reduced recurrence-free survival (HR: 10.14; 95%: CI: 1.07 -1347.9; p = 0.03). ctDNA findings were found to correlate and precede imaging results. However, CA 19-9 and CEA, in certain cases showed discordance with imaging and were found to be elevated due to other conditions, such as gastritis.
In these 8 cases of resectable pancreatic/ampullary adenocarcinoma, 7 patients received mFOLFIRINOX adjuvant chemotherapy and 2 received additional rounds of gemcitabine/oxaliplatin and gemcitabine/paclitaxel. Three patients had plasma collected after surgery, of which 2 patients were found to be ctDNA-positive and eventually relapsed. During the course of the follow-up, 50% (4/8) of patients relapsed. The presence of ctDNA was associated with reduced recurrence-free survival (HR: 10.14; 95%: CI: 1.07 -1347.9; p = 0.03). ctDNA findings were found to correlate and precede imaging results. However, CA 19-9 and CEA, in certain cases showed discordance with imaging and were found to be elevated due to other conditions, such as gastritis.
CONCLUSION
Our findings suggest, presence of ctDNA after surgery in early-stage PDAC is associated with reduced recurrence-free survival. During monitoring, ctDNA was found to be a better prognostic marker compared to CA-19 9 and CEA and can be used to inform on disease status prior to imaging.
Our findings suggest, presence of ctDNA after surgery in early-stage PDAC is associated with reduced recurrence-free survival. During monitoring, ctDNA was found to be a better prognostic marker compared to CA-19 9 and CEA and can be used to inform on disease status prior to imaging.
REFERENCES
- American Cancer Society. Cancer Facts & Figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Lee J, Ahn S, Cho IK, et al. Management of recurrent pancreatic cancer after surgical resection: a protocol for systematic review, evidence mapping and meta-analysis.BMJ Open 2018;8:e017249.
- Loft M, Lee B, Tie J, et al. Clinical applications of circulating tumor DNA in pancreatic adenocarcinoma. Journal of Personalized Medicine. 2019; 18:9(3) pii: E37.
CORRESPONDING AUTHOR:
Maen Abdelrahim, MD, PhD, Pharm.B. Section of GI oncology, Department of Medical Oncology, Houston Methodist Cancer Center. Weill Cornell Medical College and Cockrell Center of Advanced Therapeutics Phase I program. 6445 Fannin, OPC-24, Houston, TX 77030 mabdelrahim@houstonmethodist.org
Maen Abdelrahim, MD, PhD, Pharm.B. Section of GI oncology, Department of Medical Oncology, Houston Methodist Cancer Center. Weill Cornell Medical College and Cockrell Center of Advanced Therapeutics Phase I program. 6445 Fannin, OPC-24, Houston, TX 77030 mabdelrahim@houstonmethodist.org
The data in this poster was presented at World Congress of Gastrointestinal Cancer 2021. Published with permission from the Copyright owner.
