1Hematology Unit, University of Siena, Azienda Ospedialiero Universitaria Senese, Siena, Italy
Introduction
Tyrosine kinase inhibitors (TKI) may offer a normal life expectancy to Chronic Myeloid Leukemia (CML) Patients. However, during treatment with nilotinib, a higher than expected incidence of arterial occlusive events (AOEs) was observed.
We retrospectively showed in "inflammatory status" during nilotinib treatment that may explain this increased incidence of AOEs [1]. Here, we report results of a prospective multicenter (KIARO) study including 186 CML patients (89 imatinib, 59 nilotinib, 38 dasatinib) in which pro/anti-inflammatory cytokines were measured at diagnosis and during treatment, with the aim to investigate potential changes in each patient's inflammatory status possibly favoring AOEs.
Tyrosine kinase inhibitors (TKI) may offer a normal life expectancy to Chronic Myeloid Leukemia (CML) Patients. However, during treatment with nilotinib, a higher than expected incidence of arterial occlusive events (AOEs) was observed.
We retrospectively showed in "inflammatory status" during nilotinib treatment that may explain this increased incidence of AOEs [1]. Here, we report results of a prospective multicenter (KIARO) study including 186 CML patients (89 imatinib, 59 nilotinib, 38 dasatinib) in which pro/anti-inflammatory cytokines were measured at diagnosis and during treatment, with the aim to investigate potential changes in each patient's inflammatory status possibly favoring AOEs.
AIM
The aims of this study: 1) to analyze prospectively inflammation status during TKI treatment; 2) to recored AOEs; 3) to calculate the SCORE and evaluate its predictive role for AOEs; 4) to analyze possible associations of AOEs with altered inflammation status.
The aims of this study: 1) to analyze prospectively inflammation status during TKI treatment; 2) to recored AOEs; 3) to calculate the SCORE and evaluate its predictive role for AOEs; 4) to analyze possible associations of AOEs with altered inflammation status.
Methods
Inflammatory status was evaluated by measuring IL6, IL10, TNFa, oxLDL and hs-CRP plasma levels at diagnosis and during treatment ( +1, +3, +6, +12 months); additionally, clinical and biochemical pro-atherothrombotic profiles and the 10-year SCORE chart were also evaluated.
Inflammatory status was evaluated by measuring IL6, IL10, TNFa, oxLDL and hs-CRP plasma levels at diagnosis and during treatment ( +1, +3, +6, +12 months); additionally, clinical and biochemical pro-atherothrombotic profiles and the 10-year SCORE chart were also evaluated.
Results
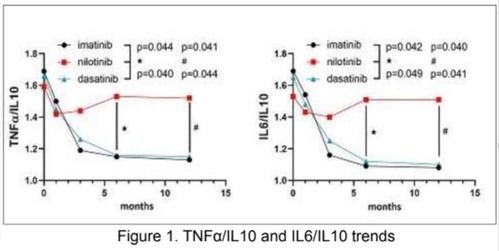
186 newly-diagnosed CML patients starting either imatinib, nilotinib or dasatinib treatment, entered this study. Regarding the inflammation status, we observed that TNFa dn IL6 levels were high at diagnosis and decreased during the first 12 months of treatment regardless the type of TKI; instead, IL10 levels were comparable among the 3 TKI cohorts at baseline, but showed a remarkably different evolution during treatment. In fact, IL10 levels were significantly higher after 6 and 12 months of imatinib (p=0.012 and p=0.009, respectively) and dasatinib (p=0.032 and p=0.014 at 12 months) compared to nilotinib. Consequently, TNFa/IL10 ratio was significantly higher in nilotinib cohort at 6 and 12 months respect to imatanib (p=0.044 at 6 months and p=0.041 at 12 months) and dasatinib (p=0.040 at 6 months and p=0.044 at 12 months). as well IL6/IL10 ratio was significantly higher in nilotinib cohort comparted to imatinib and dasatinib both at 6 (p=0.042 and p=0.049, respectively) and 12 months (p= 0.040 and p=0.041, respectively) (Figure 1). OxLDL leveles were similar in the 3 groups for the first 6 months. at 12 months we dectected a significant increaseof oxLDL levels in the nilotinib cohort (p-0.041), respect to imatinib and dasatinib. We did not find significant difference in hs-CRP levels across the 3 TKIs, although a trend for higher levels was observed in Nilotinib cohort.
Overall, these results suggest a TKI-driven pro-inflammatory status in nilotinib treated patients.
186 newly-diagnosed CML patients starting either imatinib, nilotinib or dasatinib treatment, entered this study. Regarding the inflammation status, we observed that TNFa dn IL6 levels were high at diagnosis and decreased during the first 12 months of treatment regardless the type of TKI; instead, IL10 levels were comparable among the 3 TKI cohorts at baseline, but showed a remarkably different evolution during treatment. In fact, IL10 levels were significantly higher after 6 and 12 months of imatinib (p=0.012 and p=0.009, respectively) and dasatinib (p=0.032 and p=0.014 at 12 months) compared to nilotinib. Consequently, TNFa/IL10 ratio was significantly higher in nilotinib cohort at 6 and 12 months respect to imatanib (p=0.044 at 6 months and p=0.041 at 12 months) and dasatinib (p=0.040 at 6 months and p=0.044 at 12 months). as well IL6/IL10 ratio was significantly higher in nilotinib cohort comparted to imatinib and dasatinib both at 6 (p=0.042 and p=0.049, respectively) and 12 months (p= 0.040 and p=0.041, respectively) (Figure 1). OxLDL leveles were similar in the 3 groups for the first 6 months. at 12 months we dectected a significant increaseof oxLDL levels in the nilotinib cohort (p-0.041), respect to imatinib and dasatinib. We did not find significant difference in hs-CRP levels across the 3 TKIs, although a trend for higher levels was observed in Nilotinib cohort.
Overall, these results suggest a TKI-driven pro-inflammatory status in nilotinib treated patients.
Figure 1
After a median follow-up of 23.3 months of TKI treatment, 10 patients (5.4%) suffered an AOE, specifically: 6 ACS, 2 PAOD, 1 TIA and 1 stroke. 5 events (50%) occurred in patients treated with nilotinib, either in first line (4 patients) or in third line (1 patient, after failure following brief treatment with imatinib and dasatinib). In this subgroup of 10 patientsexperiencing an AOE, we observed a trend of increased IL6 and TNFa Median values both at diagnosis and at each time point, compared with the remaining no-AOE patients. IL10 and oxLDL had similar median concentrations in both AOE and no-AOE cohorts, except for oxLDL at 12 months which resulted higher in patients who experienced AOEs. Moreover, regarding AOEs, nilotinib treatment showed a 3.1 times increased risk compared to other TKIs (HR 3.1, 95% CI 2.6-4.4 p<0.001), whereas 10-year SCORE was not predictive in the whole cohort (HR 0.6 95% CI 0.33-0.94 p=0.094) or in any subgroup (imatinib HR 0.8, 95% CI 0.49-1.03 p=0.067; nilotinib HR 0.4, 95% CI 0.29-0.76 p=0.112, dasatinib HR 0.6, 95% CI 0.37-0.92 p=0.082).
Conclusions
Our results showed a pro-inflammatory/oxidative milieu increasing along treatment with nilotinib compared with imatinib or dasatinib, as demonstrated by higher IL6/IL10 and TNFa/IL10 ratios, higher levels of oxLDL and a trend for higher hs-CRP only in nilotinib cohort. However, due to the low number of observed events, a formal statistical analysis for any association between AOEs and Pro/anti-inflammatory cytokines levels was not possible. Therefore, a longer follow-up is needed to further confirm the active role of nilotinib in AOEs pathogenesis.
Our results showed a pro-inflammatory/oxidative milieu increasing along treatment with nilotinib compared with imatinib or dasatinib, as demonstrated by higher IL6/IL10 and TNFa/IL10 ratios, higher levels of oxLDL and a trend for higher hs-CRP only in nilotinib cohort. However, due to the low number of observed events, a formal statistical analysis for any association between AOEs and Pro/anti-inflammatory cytokines levels was not possible. Therefore, a longer follow-up is needed to further confirm the active role of nilotinib in AOEs pathogenesis.
REFERENCES
1) Bocchia M, Galiberti S, Aprile L, Sicuranza A, Gozzini A, Santilli F, et al. Genetic predisposition and induced pro-inflammatory/pro-oxidatvie status may play a role in increased atherothrombotic events in nilotinib treated chronic myeloid leukemia patients. Oncotarget. 2016 Nov1; 7(44): 72311-72321
1) Bocchia M, Galiberti S, Aprile L, Sicuranza A, Gozzini A, Santilli F, et al. Genetic predisposition and induced pro-inflammatory/pro-oxidatvie status may play a role in increased atherothrombotic events in nilotinib treated chronic myeloid leukemia patients. Oncotarget. 2016 Nov1; 7(44): 72311-72321
The data in this poster was presented at ASH 2021. Published with permission from the Copyright owner.
