1Molecular Partners AG, Zurich-Schlieren, Switzerland
Abstract
AML is driven by leukemic stem cells (LSCs) that resist conventional chemotherapies and remain unaffected in their niche, continually replenishing circulating blast cells. We postulated that an avidity-engineered CD3engaging DARPin (Designed Ankyrin Repeat Protein), able to simultaneously target LSC-specific CD70 as well as CD123 and CD33, could allow highly efficient and specific T cell-mediated killing of AML LSCs and circulating blast cells while preserving a therapeutic window towards healthy cells. Moreover, this simultaneous targeting of three different tumor associated antigens (TAAs) has the potential to address tumor heterogeneity, allowing targeting of AML cells with different co-expression patterns and/or expression levels of each single TAA. To achieve this ambitious goal, we used our DARPin platform to build a novel class of triple targeting CD3 engaging molecules.
AML is driven by leukemic stem cells (LSCs) that resist conventional chemotherapies and remain unaffected in their niche, continually replenishing circulating blast cells. We postulated that an avidity-engineered CD3engaging DARPin (Designed Ankyrin Repeat Protein), able to simultaneously target LSC-specific CD70 as well as CD123 and CD33, could allow highly efficient and specific T cell-mediated killing of AML LSCs and circulating blast cells while preserving a therapeutic window towards healthy cells. Moreover, this simultaneous targeting of three different tumor associated antigens (TAAs) has the potential to address tumor heterogeneity, allowing targeting of AML cells with different co-expression patterns and/or expression levels of each single TAA. To achieve this ambitious goal, we used our DARPin platform to build a novel class of triple targeting CD3 engaging molecules.
Introduction
In AML, medical need remains high. The treatment of relapsed or refractory (r/r) AML is challenging due to the heterogeneous nature of the disease and to high relapse rates with current standard-of-care [1]. Various highly potent, single-targeting T-cell engager (TCE) and CAR-T therapies have entered clinical development but are often accompanied by dose limiting toxicities (DLTs), such as cytokine release syndrome (CRS) and myelotoxicities, that exclude robust anti-tumor efficacy [1][2].
More selective therapies and rationally designed target combinations are desperately needed to allow for extended dose escalation with a more acceptable safety profile to achieve durable responses.
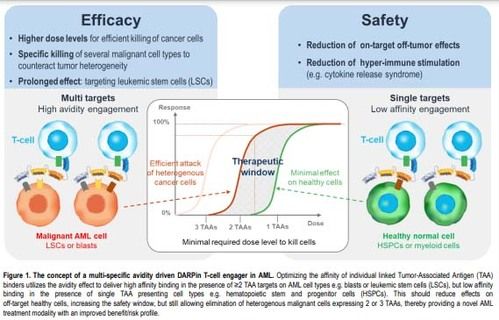
To overcome these limitations, we leveraged our proprietary DARPin platform to screen multi-specific CD3 engaging DARPin molecules with optimal target affinity and molecular architecture to ensure potent avidity-driven T cell-mediated killing AML cells with an improved safety profile (figure1).
In AML, medical need remains high. The treatment of relapsed or refractory (r/r) AML is challenging due to the heterogeneous nature of the disease and to high relapse rates with current standard-of-care [1]. Various highly potent, single-targeting T-cell engager (TCE) and CAR-T therapies have entered clinical development but are often accompanied by dose limiting toxicities (DLTs), such as cytokine release syndrome (CRS) and myelotoxicities, that exclude robust anti-tumor efficacy [1][2].
More selective therapies and rationally designed target combinations are desperately needed to allow for extended dose escalation with a more acceptable safety profile to achieve durable responses.
To overcome these limitations, we leveraged our proprietary DARPin platform to screen multi-specific CD3 engaging DARPin molecules with optimal target affinity and molecular architecture to ensure potent avidity-driven T cell-mediated killing AML cells with an improved safety profile (figure1).
Methods and Materials
Our DARPin libraries contain trillions of molecules allowing the generation of highly diverse binders against target proteins that can be easily combined into multi-specific DARPin to elicit desired biological effects. The Tumor Associated Antigens (TAAs) selected for our multi-specific DARPin TCE MP0533 are CD33, CD123, and CD70. They are clinically validated targets in AML;
Our DARPin libraries contain trillions of molecules allowing the generation of highly diverse binders against target proteins that can be easily combined into multi-specific DARPin to elicit desired biological effects. The Tumor Associated Antigens (TAAs) selected for our multi-specific DARPin TCE MP0533 are CD33, CD123, and CD70. They are clinically validated targets in AML;
- CD33 is expressed on ~80-90% of AML blasts and on LSCs [3][4]
- CD123 is expressed on ~70-80% of AML blasts and is more specific for LSCs than CD33 [5][6]
Results
We leveraged our proprietary DARPin platform to screen multi-specific CD3engaging DARPin molecules, including serum albumin binding DARPin for systemic half-life extension, and indentify the optimal target affinity and colecular architecture to ensure potent avidity-driven T cell-mediated killing of AML cells while sparing healthy cells.
This approach allowed the generation of CD3-engaging DARPins able to target simultaneously CD33, CD123, and CD70. Such DARPins demonstrated potency, in both allogeneic and autologous setting, against AML cell lines and primary cells expressing any combination of at least 2 of the 3 targeted TAAs, while showing low activity against single TAA-expressing cells, the latter representing cells of the healthy compartment. Higher expression of the selected TAAS on LSCs vs. normal hematopoietic stem cells (HSC) can further enhance the selectivity of such an avidity driven molecule, leading to the preferential killing of LSC over HSC.
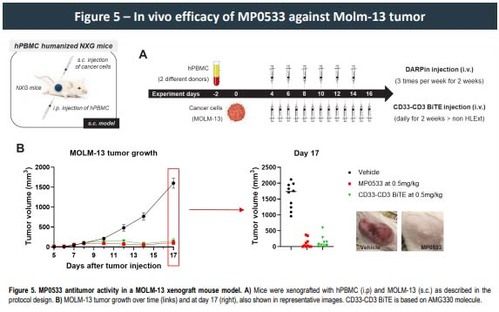
Moreover, our multi-specific T cell engager (TCE) format resulted in a significant decrease in cytokine release both in vitro and in a whole blood test system for cytokine release syndrome (CRS) when compared to other mono-targeting TCE therapies., confirming its specificity and the potential for an improved safety profile within the normal hematopoietic system. Additionally, while showing similar anti-tumor efficacy in a mouse xenograft model using Molm-13 cell line and human PBMC's, CRS measured in serum 4 h after the initial injection for our multi-specific DARPin molecule was drastically reduced compared to a reference CD33 TCE, further strengthening the evidence that our multi targeting DARPins might also exhibit a good safety profile in humans.
In summary, we were able to to generate multi-specific CD3 engaging DARPin molecules with tailored affinities towards different TAAs showing exceptional efficacy and with the potential for superior safety over mono-specific TCE approaches, including systemic half-life extension to avoid a continuous intravenous infusion-based therapy.
We leveraged our proprietary DARPin platform to screen multi-specific CD3engaging DARPin molecules, including serum albumin binding DARPin for systemic half-life extension, and indentify the optimal target affinity and colecular architecture to ensure potent avidity-driven T cell-mediated killing of AML cells while sparing healthy cells.
This approach allowed the generation of CD3-engaging DARPins able to target simultaneously CD33, CD123, and CD70. Such DARPins demonstrated potency, in both allogeneic and autologous setting, against AML cell lines and primary cells expressing any combination of at least 2 of the 3 targeted TAAs, while showing low activity against single TAA-expressing cells, the latter representing cells of the healthy compartment. Higher expression of the selected TAAS on LSCs vs. normal hematopoietic stem cells (HSC) can further enhance the selectivity of such an avidity driven molecule, leading to the preferential killing of LSC over HSC.
Moreover, our multi-specific T cell engager (TCE) format resulted in a significant decrease in cytokine release both in vitro and in a whole blood test system for cytokine release syndrome (CRS) when compared to other mono-targeting TCE therapies., confirming its specificity and the potential for an improved safety profile within the normal hematopoietic system. Additionally, while showing similar anti-tumor efficacy in a mouse xenograft model using Molm-13 cell line and human PBMC's, CRS measured in serum 4 h after the initial injection for our multi-specific DARPin molecule was drastically reduced compared to a reference CD33 TCE, further strengthening the evidence that our multi targeting DARPins might also exhibit a good safety profile in humans.
In summary, we were able to to generate multi-specific CD3 engaging DARPin molecules with tailored affinities towards different TAAs showing exceptional efficacy and with the potential for superior safety over mono-specific TCE approaches, including systemic half-life extension to avoid a continuous intravenous infusion-based therapy.
Figure 1
Conclusions
- Our unique modular DARPin platform enables the generation of affinity tailored, multi-specific, avidity driven molecules.
- MP0533, an avidity-driven multi-specific DARPin TCE, shows high potency of AML cell lines expressing at least 2 TAA, and both selectivity and efficacy windows towards cells expressing 1 TAA only.
- MP0533 also potently kills primary AML cells, both in allogenic and autologous setting, and shows a therapeutic window by preferentially killing AML LSC over healthy HSC.
- Ex-vivo whole blood and in-vitro tumor cell killing assays show a preferential safety profile of MP0533 toward cytokine secretion and cell depletion (platelet and WBC), supporting an improved therapeutic window of MP0533.
- MP0533 demonstrates anti-tumor activity in human PBMC-reconstituted mice.
- Our data supports the development of MP0533 as a unique therapeutic solution for the treatment of AML, potentially able to tackle the dose limiting toxicities of TCEs in the clinic.
Figure 2
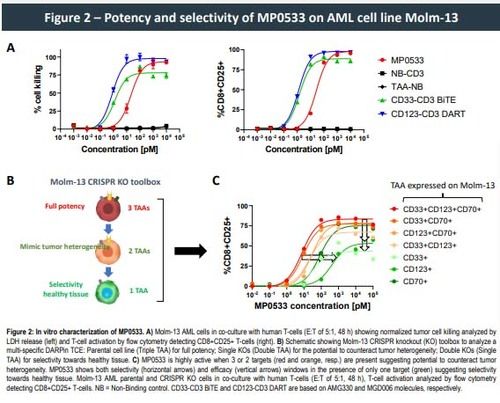
Figure 3
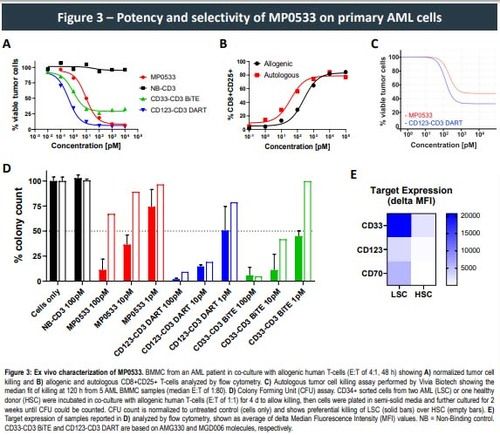
Figure 4
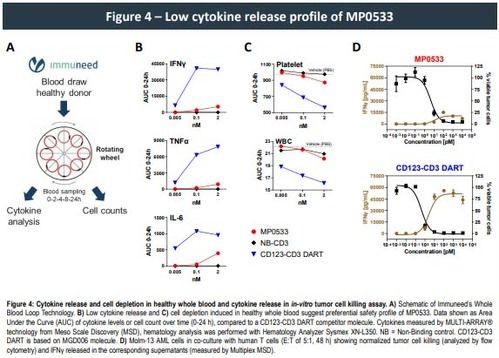
Figure 5
REFERENCES
1)Daver et. al. Blood Cancer J. (2020)
2) Guy et al. Curr Hematol Malig Rep. (2018)
3) Ehninger et al. Blood Cancer J. (2014)
4) Haubner et al. Leukemia (2019)
5) Perna et al. Cancer Cell (2017)
6) Riether et al. Nat Med (2020)
1)Daver et. al. Blood Cancer J. (2020)
2) Guy et al. Curr Hematol Malig Rep. (2018)
3) Ehninger et al. Blood Cancer J. (2014)
4) Haubner et al. Leukemia (2019)
5) Perna et al. Cancer Cell (2017)
6) Riether et al. Nat Med (2020)
The data in this poster was presented at ASH 2021. Published with permission from the Copyright owner.
